��Ŀ����
��A��B��C��D��E����ԭ���������������Ԫ��(ԭ��������С��30)��A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ�Eԭ���������1�������ӣ���������3���ܼ��Ҿ��������ӣ�D��Eͬ���ڣ��۵�����Ϊ2����
(1)D��Ԫ�ط���Ϊ______��A�ĵ��ʷ����Цм��ĸ���Ϊ______��
(2)BԪ�ص��⻯��ķе���ͬ��Ԫ���⻯������ߵģ�ԭ����__________________________________��
(3)A��B��C 3��Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
(4)д����̬Eԭ�ӵļ۵����Ų�ʽ��__________________��
(5)A������⻯����ӵĿռ乹��Ϊ________������Aԭ�ӵ��ӻ�������________��
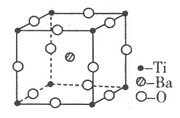
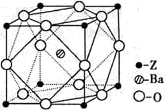
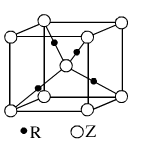
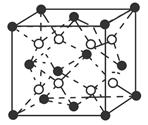
(6)C��D�γɵĻ�����ľ����ṹ��ͼ��ʾ����֪������ܶ�Ϊ�� g��cm��3�������ӵ�����ΪNA�����߳�a��________cm��(�æѡ�NA�ļ���ʽ��ʾ)
(1)D��Ԫ�ط���Ϊ______��A�ĵ��ʷ����Цм��ĸ���Ϊ______��
(2)BԪ�ص��⻯��ķе���ͬ��Ԫ���⻯������ߵģ�ԭ����__________________________________��
(3)A��B��C 3��Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
(4)д����̬Eԭ�ӵļ۵����Ų�ʽ��__________________��

(5)A������⻯����ӵĿռ乹��Ϊ________������Aԭ�ӵ��ӻ�������________��
(6)C��D�γɵĻ�����ľ����ṹ��ͼ��ʾ����֪������ܶ�Ϊ�� g��cm��3�������ӵ�����ΪNA�����߳�a��________cm��(�æѡ�NA�ļ���ʽ��ʾ)
(1)Ca��2
(2)H2O���Ӽ�������
(3)F��N��O
(4)3d104s1
(5)������sp3
(6)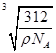
(2)H2O���Ӽ�������
(3)F��N��O
(4)3d104s1
(5)������sp3
(6)
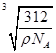
�������⼰�����Ų�ʽ�Ĺ����жϿ�֪��Ԫ��A��B��C��D��E�ֱ���N��O��F��Ca��Cu��(1)A������N2����ṹʽ��NN�����������м���(2)����H2O�ķ��Ӽ��������ʹ����е���ͬ��Ԫ���⻯������ߵġ�(3)N��2p�ܼ��ǰ����״̬���Ƚ��ȶ������һ�����ܱ�OҪ����ͬ���ڵ�FҪС��(5)NH3�Ŀռ乹���������Σ�����N��sp3�ӻ���(6)���ݾ����Ľṹ��Ӧ�á���̯��������ɵã�1�������к���4��Ca2����8��F���������ܶ�Ϊ�ѣ�(312/NA)��a3�����a�� 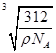 ��
��
�㲦�����⿼�����ʽṹ���ƶϣ����鿼��Ӧ����ѧ֪ʶ�����������������Ѷ��еȡ�
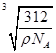 ��
���㲦�����⿼�����ʽṹ���ƶϣ����鿼��Ӧ����ѧ֪ʶ�����������������Ѷ��еȡ�

��ϰ��ϵ�д�
�����Ŀ
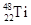 ��
��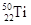 ����ԭ�ӣ����ǻ���Ϊ ���ѻ�̬ԭ�ӵĵ����Ų�ʽΪ ��
����ԭ�ӣ����ǻ���Ϊ ���ѻ�̬ԭ�ӵĵ����Ų�ʽΪ ��