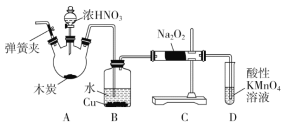
��Ŀ����
����Ŀ����ͭ�Ͻ�㷺Ӧ���ں��չ�ҵ������ͭ�Ͻ���и�����л��������Ʊ�CuAlO2������
���¡�
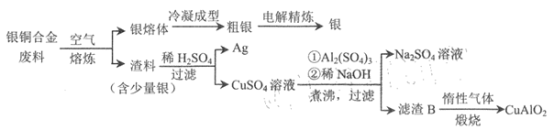
��֪��Al(OH) 3��Cu(OH) 2��ʼ�ֽ���¶ȷֱ�Ϊ450��� 80�档 ����˵���������
A.��⾫��ʱ������������������������
B.Ϊ���ԭ�������ʣ�������Ӧ�ӹ�����ϡNaOH
C.����B����ʱ�����ķ�ӦΪ 4CuO+4Al(OH)3![]() 4CuA1O2+ O2��+6H2O
4CuA1O2+ O2��+6H2O
D.���� 1.0 kg ��ͭ�Ͻ�ͭ����������Ϊ64%) �������� 10.0mol CuA1O2
���𰸡�B
��������
A. ��⾫��ʱ�����������������������������Һѡ���������Σ������������������õĽ����ܽ⣬�����ϵ��Һ�������ӵõ����ӱ���ԭ��������A��ȷ��
B. ����������ͭ��Һ�м���������ϡNaOH��δ���֮ǰ��Cu( OH) 2 �� A l(OH) 3 ������ Al(OH) 3 �� Cu(OH) 2 ��ʼ�ֽ���¶ȷֱ�Ϊ 450 �� �� 80 �� ��֪ B Ϊ Al(OH) 3 �� CuO�����ɹ��� B �Ĺ����У������ NaOH �ļ��������� NaOH �����������������ķ�ӦΪ��Al(OH) 3 ��OH ��= AlO2-��2H 2 O ,B����
C. ����BΪAl(OH) 3�� CuO�Ļ�������ʱͭ���ϼ۽��͵�+1�ۣ�����������ԭ��Ӧ����Ӧ����ʽΪ4CuO+4Al(OH)3![]() 4CuA1O2+ O2��+6H2O��C��ȷ��
4CuA1O2+ O2��+6H2O��C��ȷ��
D. 1.0 kg ��ͭ�Ͻ�ͭ����������Ϊ64%)��ͭ�����ʵ���Ϊ ![]() ������ͭԪ���غ�֪�������� 10.0mol CuA1O2��D��ȷ��
������ͭԪ���غ�֪�������� 10.0mol CuA1O2��D��ȷ��
��ѡB��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��Ϊ̽��H2O2��SO2��Br2�����ʣ�ijС��ͬѧ�������ʵ��(�гּ�β������װ������ȥ���������Ѽ���)��
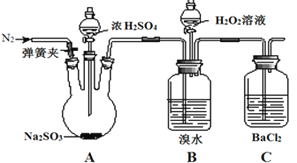
ʵ����� | ʵ������ |
i�����ɼУ�ͨ��N2һ��ʱ�䣬�رյ��ɼУ���A�з�Һ©���������μ�Ũ���� | A�������ݲ�����B�к���ɫ��ˮ��ɫ��C���а�ɫ���� |
����ȡC�г����������� | C�а�ɫ�������ܽ� |
������B�з�Һ©����������εμ�H2O2 | ��ʼʱ��ɫ�����Ա仯�������μ�H2O2��Һ��һ��ʱ����Һ��ɺ���ɫ |
(1)�ڵ���Ũ����֮ǰҪͨ��N2һ��ʱ���Ŀ����__________________________��
(2)��ʵ�����֤��SO2����_________�ԣ�H2O2��������_________Br2(�ǿ�ڡ������ڡ�)��
(3)B�к���ɫ��ȥ��ԭ��_______________________________��B�����±�ɺ���ɫ��ԭ��____________________________________(�������ӷ���ʽ��ʾ)��
(4)C�в����İ�ɫ������______��
(5)��ͬѧͨ��C�в�����ɫ�������ó����ۣ�SO2����BaCl2��Ӧ������������ͬѧ��Ϊ���ܵó��˽��ۣ�����ʵ������˸Ľ�����B��C֮������ʢ��_________��ϴ��ƿ���ٴν���ʵ��ʱ����C��δ����������
(6)Ϊ��һ���о�SO2�����ʣ��ֱ�����к�δ��й�������ˮ����Ba(NO3)2��BaCl2��Һ����������ʵ�飺
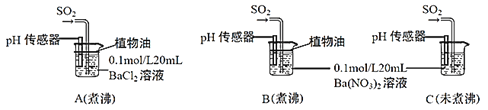
��ʵ��A�У�û�й۲쵽��ɫ��������pH��������ʾ��Һ�����ԣ�ԭ����__________________��(�û�ѧ����ʽ��ʾ)
��ʵ��C�г��ְ�ɫ������ʵ��B��ܶࡣ�ɴ˵ó��Ľ�����__________________����ʵ��B��C��ͨ��������SO2����Һ���ԣ�B_________C(�ǿ�ڡ������ڡ������ڡ�)��
(7)Na2SO3���������Ų����������ʡ���ȡ��Ʒ1.8������ˮ����ҺA����A�м�������BaCl2��Һ,���˵ó���B��������B����������ϡ�����,������������ʧ������������0.16g(���������ȫת��)����Ʒ��Na2SO3������������___________________��