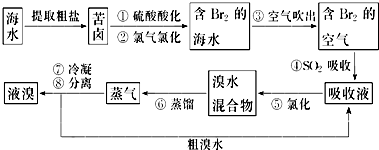
��Ŀ����
18��ԭ��������������Ķ�����Ԫ��a��b��c��d��e�У�a������������Ϊ���������Ķ�����b��d��A2B���⻯���ΪV�η��ӣ�c��+1�����ӱ�e��-1��������8�����ӣ��ش��������⣺
��1��Ԫ��cΪNa��dΪS
��2������ЩԪ���γɵ�˫ԭ�ӷ���ΪCO��O2��Cl2��
��3����ЩԪ�صĵ��ʻ��������γɵ�AB�ͻ������У��侧����������ԭ�Ӿ�����ǽ��ʯ�����Ӿ������NaCl�������������Na�����Ӿ������CO����O2��Cl2������ÿ����һ�֣�
��4��Ԫ��a��b�γɵ�һ�ֻ�������c��b�γɵ�һ�ֻ�������ķ�Ӧ�����ڷ�������У����ֻ�����ĵ���ʽ�ֱ�Ϊ��
 ��
�� ���÷�Ӧ�Ļ�ѧ����ʽΪ��2CO2+Na2O2�T2Na2CO3+O2��
���÷�Ӧ�Ļ�ѧ����ʽΪ��2CO2+Na2O2�T2Na2CO3+O2��
���� ԭ��������������Ķ�����Ԫ��a��b��c��d��e�У�a������������Ϊ���������Ķ�������a��2�����Ӳ㣬����������Ϊ4����aΪ̼Ԫ�أ�b��d��A2B���⻯���ΪV�η��ӣ�B��ȡsp3�ӻ���������Bԭ����2�Թ¶Ե��ӣ���Bԭ������������Ϊ6�����ڢ�A�壬��bΪ��Ԫ�أ�dΪ��Ԫ�أ�c��+1�����ӱ�e��-1��������8�����ӣ�c���ڢ�A�塢e����VIIA�壬���ԭ��������֪��cΪ��Ԫ�أ�eΪClԪ�أ�
��� �⣺ԭ��������������Ķ�����Ԫ��a��b��c��d��e�У�a������������Ϊ���������Ķ�������a��2�����Ӳ㣬����������Ϊ4����aΪ̼Ԫ�أ�b��d��A2B���⻯���ΪV�η��ӣ�B��ȡsp3�ӻ���������Bԭ����2�Թ¶Ե��ӣ���Bԭ������������Ϊ6�����ڢ�A�壬��bΪ��Ԫ�أ�dΪ��Ԫ�أ�c��+1�����ӱ�e��-1��������8�����ӣ�c���ڢ�A�塢e����VIIA�壬���ԭ��������֪��cΪ��Ԫ�أ�eΪClԪ�أ�
��1��������������֪��Ԫ��cΪNa��dΪS���ʴ�Ϊ��Na��S��
��2������ЩԪ���γɵ�˫ԭ�ӷ���ΪCO��O2��Cl2���ʴ�Ϊ��CO��O2��Cl2��
��3����ЩԪ�صĵ��ʻ��������γɵ�AB�ͻ������У��侧����������ԭ�Ӿ�����ǽ��ʯ���������Ӿ������NaCl�ȣ����ڽ����������Na�����ڷ��Ӿ������CO����O2��Cl2����
�ʴ�Ϊ�����ʯ��NaCl��Na��CO����O2��Cl2����
��4��Ԫ��a��b�γɵ�һ�ֻ�������c��b�γɵ�һ�ֻ�������ķ�Ӧ�����ڷ�������У�Ӧ��CO2��Na2O2�����ֻ�����ĵ���ʽ�ֱ�Ϊ�� ��
�� ���÷�Ӧ�Ļ�ѧ����ʽΪ��2CO2+Na2O2�T2Na2CO3+O2��
���÷�Ӧ�Ļ�ѧ����ʽΪ��2CO2+Na2O2�T2Na2CO3+O2��
�ʴ�Ϊ�� ��
�� ��2CO2+Na2O2�T2Na2CO3+O2��
��2CO2+Na2O2�T2Na2CO3+O2��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ����ǽ���ؼ����漰��������Ų������ӽṹ���������͡�����ʽ�ȣ��Ѷ��еȣ�ע��Ի���֪ʶ�����գ�

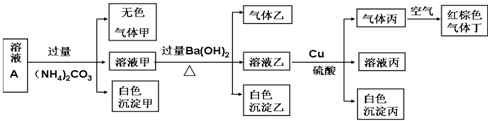
| A�� | ����Һ��һ�������������е�NO3-��Al3+��SO42-��Cl-�������� | |
| B�� | ʵ������Cu 14.4g�����������嶡�����Ϊ3.36L | |
| C�� | ������һ����BaCO3��������BaSO4 | |
| D�� | һ��û��Fe3+��������ȷ���Ƿ���I- |
| A�� | ����ˮ���������ڻ���� | B�� | �����ܶȱ�ˮ�� | ||
| C�� | �����������ľ����������� | D�� | ����ڱ�����ṹ����ؼ����� |
| A�� | 1molNH4+ ����������Ϊ10NA | |
| B�� | ���³�ѹ�£�48gO2��O3�������������ԭ����Ϊ3NA | |
| C�� | 22.4LSO2�����ķ�����ΪNA | |
| D�� | ͬ��ͬѹ�£������ͬ�����������������ķ�������� |
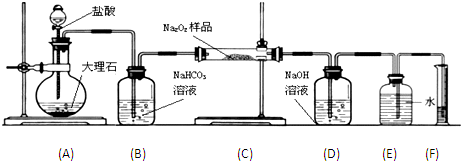
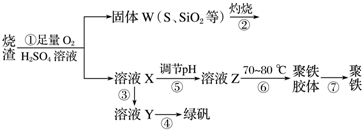

 ��
�� CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��