��Ŀ����
14��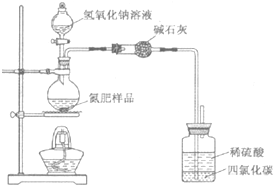 ij����������л���������泥�Ϊ�ⶨ�õ��ʵĺ�������һ��ѧ�о���ѧϰС��ȡһ����������Ʒ����ĥʹ���Ͼ��ȣ����ã���֪��
ij����������л���������泥�Ϊ�ⶨ�õ��ʵĺ�������һ��ѧ�о���ѧϰС��ȡһ����������Ʒ����ĥʹ���Ͼ��ȣ����ã���֪��������������ʱ2NH4HSO4+2NaOH=��NH4��2SO4+Na2SO4+2H2O
�������ƹ���ʱNH4HSO4+2NaOH=Na2SO4+NH3��+2H2O
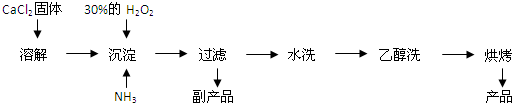
��1��ͬѧ����Ƶ�ʵ��װ��ͼ���£���ش�������⣮
��װ���м�ʯ�ҵ����������հ����е�ˮ������
�ڹ��ƿ�����Ȼ�̼�������Ƿ�ֹ������
��ָ����ʦָ�����ø�װ�ý���ʵ�飬��ʹ��������������ʵ��������ް������ݣ���õ���Ʒ�������Խ�ƫ�ͣ���ԭ������Dz����ڷ�Ӧװ���еİ���δ��ϡ�������գ�
��2��ͬѧ�ǸĽ�ʵ��װ�úĽ�װ��ͼ�ԣ�����������ʵ�飮��ȡ��ͬ������������Ʒ�ֱ���40.00mL��ͬŨ�ȵ�����������Һ��ϣ���ȫ�ܽ���ȳ�ַ�Ӧ�����¶�����β��ֽ⣩����ʹ���ɵİ���ȫ����ϡ�������գ���ð���������������ʵ���������£�
| ����������Һ/mL | 40.00 | ||
| ��Ʒ����/g | 7.750 | 15.50 | 23.25 |
| ��������/g | 1.870 | 1.870 | 1.700 |
����Ʒ�е�Ԫ�ص�����������19.87%��
����������������Һ�����ʵ���Ũ��Ϊ3.25 mol/L��
������Ʒ����Ϊ31.00g�������ɰ���������Ϊ1.53 g��
���� ��1���ټ�ʯ�����ڸ��ﰱ����
�ڰ�����������ˮ����������������
�۷�Ӧ���ɵİ������ܹ���ϡ������ȫ���գ�
��2���ٸ���ʵ�����ݣ��жϳ���һ��ʵ�����������ƹ��������е�����Ӷ������˰��������ݵ�ԭ���غ���ʽ�������Ʒ�е�Ԫ�ص�����������
�ڸ��ݵ�һ�����ݼ��������狀�������淋ĺ������ٸ��ݵڶ��������е��������ƶ������˷�Ӧ�����������Ʋ��㣬������������Ƶ�Ũ�ȣ�
�۸����������Ƶ����ʵ�������Ʒ���������Ƶ����ʵ�������������ɵİ��������ʵ�����������
��� ��1�������ɵİ����к���ˮ�֣��ü�ʯ�����հ����е�ˮ�֣�
�ʴ�Ϊ�����հ����е�ˮ������
�ڰ�����������ˮ�����������������Թ��ƿ�����Ȼ�̼���ڷ�ֹ������
�ʴ�Ϊ����ֹ������
���ø�װ�ý���ʵ�飬��ʹ��������������ʵ��������ް�����й������װ���ڵİ���������ȫ��ϡ�������գ���õ���Ʒ�������Խ�ƫ�ͣ�
�ʴ�Ϊ�������ڷ�Ӧװ���еİ���δ��ϡ�������գ�
��2�����赪������淋���������ΪX����������淋���������Ϊ��1-X����
��ʵ�鷽�������ݿ�������һ��ʵ��͵ڶ���ʵ�������ɵİ���������ͬ��˵����һ��ʵ�������������ǹ����ģ���������Ӷ������˰��������ݵ�Ԫ���غ㣬$\frac{7.75��X}{132}$��17+$\frac{7.75����1-X��}{115}$=1.87
��ã�X=0.85��
������������淋���������Ϊ85%��������淋���������Ϊ15%��
���ݵ�ԭ���غ㣺��ԭ�ӵ����ʵ����͵��ڰ��������ʵ�����
������Ʒ�е�Ԫ�ص���������Ϊ��$\frac{\frac{1.87}{17}��14}{7.75}$��100%=19.87%��
�ʴ�Ϊ��19.87��
�ڼ���ڶ���ʵ�鷽�������������ǹ����ģ������ɰ�������Ϊ��$\frac{15.50��0.85}{132}$+$\frac{15.50��0.15}{115}$��3.74g��1.87g��
��˵���ڶ���ʵ�鷽���У��������Ƶ����ʵ������㣬�������Ʒ�Ӧ����������е������Ӻ�ȫ����������泥�ʣ������������Ӳ����Խ�ȫ��笠����ӷ�Ӧ���������ֻ��Ӧ��һ��������泥��ų���1.87g������
���ݷ�Ӧ����ʽ��2NH4HSO4+2NaOH=��NH4��2SO4+Na2SO4+2H2O����NH4��2SO4+2NaOH=Na2SO4+2NH3��+2H2O��
������������Ƶ����ʵ���Ϊ��n��NH4HSO4��+n��NH3��=$\frac{15.5��0.15}{115}$mol+$\frac{1.87}{17}$mol��0.13mol��
����������Һ��Ũ��Ϊ��$\frac{0.13mol}{0.04L}$=3.25mol/L��
�ʴ�Ϊ��3.25��
������Ʒ����Ϊ31g�������������������ʵ�������0.13mol��
���ݷ�Ӧ����ʽ��2NH4HSO4+2NaOH=��NH4��2SO4+Na2SO4+2H2O��
��Ӧ������������ĵ��������Ƶ����ʵ���Ϊ��$\frac{31��0.15}{115}$mol=0.04mol��
ʣ�µ������������ʵ���Ϊ��0.13mol-0.04mol=0.09mol��
���ݷ�Ӧ����ʽ����NH4��2SO4+2NaOH=Na2SO4+2NH3��+2H2O�����ɵİ��������ʵ���Ϊ��0.09mol��
�������ɵİ���������Ϊ��17g/mol��0.09mol=1.53g��
�ʴ�Ϊ��1.53��
���� ���⿼���˲ⶨ����������е�Ԫ�صĺ�������Ŀ�������Ƚϴ���Ҫ����������Ӧ������ʵ�����ݣ��ó���ȷ���ۣ������ֿ�������ѧ�ķ�����������ۺϼ��������������Ѷ��Դ�

| A�� | ��ʳ�׳�ȥůˮƿ�еı���ˮ�� | |
| B�� | ����������ʳ�����Ƿ���أ�KIO3�� | |
| C�� | ҽ�þƾ���Ũ��ͨ��Ϊ75%��������ɱ������ | |
| D�� | �����ղ�����ζ�ķ���������֯��ʹ���ë֯�� |
| A�� | �����¶Ⱥ�����ѹǿ���䣬����1mol NH3��g�� | |
| B�� | �����¶Ⱥ�����������䣬����1mol NH3��g�� | |
| C�� | �����¶Ⱥ�����ѹǿ���䣬����1mol N2��g�� | |
| D�� | �����¶Ⱥ�����������䣬����1mol H2��g�� |
| A�� | ��NaAlO2��Һ��ͨ�������CO2��2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32- | |
| B�� | NH4HCO3��Һ�м����������������Һ��NH4++OH-�TNH3��H2O | |
| C�� | AlCl3��Һ�еμӹ�����ˮ��Al3++3NH3��H2O�T3NH4++Al��OH��3�� | |
| D�� | ������������Fe3O4������ϡ����3Fe2++4H++NO3-�T3Fe3++NO��+2H2O |
| A�� | ��������������ˮ�У��и�ʱҪ��ˮ�½��� | |
| B�� | Һ��ֱ�ӱ�����ĥ�ڲ�������ϸ����ɫƿ�� | |
| C�� | KOH��Һ�����ĥ�ڲ������Ĺ��ƿ�� | |
| D�� | ���������ƴ������ˮ�Ҵ��� |
| A�� | H2��ȼ���ȣ���H����285.8kJ•mol-1 | |
| B�� | ��Ӧ2H2O��g��=2H2��g��+O2��g��H2���ʱ䣨��H����571.6kJ•mol-1 | |
| C�� | ��Ӧ2H2O��l��=2H2��g��+O2��g��ֻ���ڵ�������½��� | |
| D�� | ��Ӧ2H2O��l��=2H2��g��+O2��g����һ�������¿����Է����� |
| A�� | ���³�ѹ�£�18 gˮ�к��е�ˮ������ĿΪNA | |
| B�� | ���³�ѹ�£�1.06g Na2CO3���е�Na+������Ϊ0.02 NA | |
| C�� | ͨ��״���£�NA ��CO2����ռ�е����Ϊ22.4L | |
| D�� | ���ʵ���Ũ��Ϊ0.5mol/L��MgCl2��Һ�У�����Cl-����ΪNA |
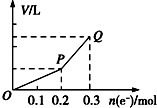 �ö��Ե缫�������ͭ��Һ����������ת�Ƶ��ӵ����ʵ������������������Ĺ�ϵ��ͼ��ʾ���������������ͬ״���²ⶨ������ʹ��Һ�ָ�����ʼ״̬��������Һ�м��루������
�ö��Ե缫�������ͭ��Һ����������ת�Ƶ��ӵ����ʵ������������������Ĺ�ϵ��ͼ��ʾ���������������ͬ״���²ⶨ������ʹ��Һ�ָ�����ʼ״̬��������Һ�м��루������| A�� | 0.15 mol CuO | B�� | 0.1 mol CuCO3 | ||
| C�� | 0.075mol Cu��OH��2 | D�� | 0.05 mol Cu2��OH��2CO3 |