��Ŀ����
ijУ��ѧ�о���ѧϰС����ѧϰ���Ҵ�����ȩ�����ʺ���������ʵ��̽����

�Իش��������⣺
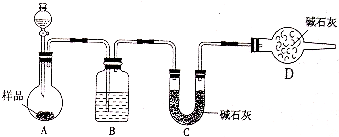
��1������ͼ���г�װ��������ʡ�ԣ������Ҵ���ͭ����������������Ӧ����ȩ��ʵ�飬��װ������װ�ã��ڼ���ҩƷǰ������Ӧ���еIJ�����______��C����ˮ��������______��
��2��ʵ�鿪ʼʱ���ȹر�ֹˮ��a����Һ©����������ͭ˿��λ����Ƭ�̣�Ȼ���ֹˮ��a���� D���۲쵽��ʵ������Ϊ______��D����Ӧ�Ļ�ѧ����ʽΪ______��
��3��ȡ��Ӧ��F�е�������Һ�������Ƶ�������ͭ��������Һ�в����������ڣ��۲쵽�к�ɫ������������ͬѧ��Ϊ��ɫ�����ijɷֿ�����Cu2O��Cu��Cu2O��Cu�Ļ���
ͨ���������ϻ�֪���������������£�Cu2O+2H+=Cu2++Cu+H2O����Cu��CuO�ڿ����и������վ�����Cu2O��
Ϊ����֤��ɫ�����ijɷ��������������ʵ�飺
����1 ȡ�����ú�ɫ�����������м���������ϡ���ᣬ�۲췴Ӧ����
����2 ȡ�����ú�ɫ�����������м���������ϡ���ᣬ�۲췴Ӧ����
����3 ��ȡ����ָ���ĸú�ɫ����a g���ڿ����и������գ����ڸ���������ȴ���ٳ��أ��������ֱ�����أ��Ƶù��������Ϊb g���Ƚ�a��b�Ĵ�С��ϵ��
���������������ܹ�ȷ���ú�ɫ�����ɷֵ���______��
������������ȷ����ԭ����______��
�⣺��1������װ��ͼ��֪�����巢��װ�����ڷ�Ӧ��ʼǰ��Ҫ���������Լ�飬C���ṩ�Ҵ��������д�������Ӧ��
�ʴ�Ϊ�����װ�õ������ԣ�����ƽ�ȵ��Ҵ�������
��2��ʵ�鿪ʼʱ���ȹر�ֹˮ��a����Һ©����������ͭ˿��λ����Ƭ�̣�������Ӧ�������������� Eװ�ü��Ⱥ�ɫͭ�仯Ϊ��ɫ����ͭ��Ȼ���ֹˮ��a���Ҵ��������������ͭ����������ȩ��ͭ�ɺ�ɫ�仯Ϊ��ɫ������ֱ�Ϊ��ɫ����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2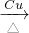 2CH3CHO+2H2O��
2CH3CHO+2H2O��
�ʴ�Ϊ����ڵ�ͭ˿�ֱ�죬���ֽ���仯��2CH3CH2OH+O2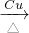 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��3������1������Cu��Cu2O������ϡ���ᷴӦ���õ���ɫ��Һ�����Է���1���У�
����2��Cu2O��ϡ���ᷴӦ��Cu���ɣ��в�����Cu����ϡ���ᷴӦ�в��������Է���2���У�
����3��ͭ�ڿ�������������CuO��Cu2O�ڿ���������Ҳ��������ͭ�����������ӵ�������ͬ��
agCu�ڿ��������գ���ȫ��Ӧ��������Ϊ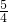 ag��agCu2O�ڿ��������գ���ȫ��Ӧ��������Ϊ
ag��agCu2O�ڿ��������գ���ȫ��Ӧ��������Ϊ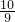 ag������ag��ɫ�����ڿ�����������ȫ��Ӧ��bΪ
ag������ag��ɫ�����ڿ�����������ȫ��Ӧ��bΪ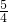 agʱ��ɫ����Cu����bΪ
agʱ��ɫ����Cu����bΪ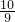 ag��ʱ��ɫ���� Cu2O����
ag��ʱ��ɫ���� Cu2O����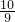 a g��b��
a g��b��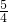 a g��ʱ��ɫ����Ϊ���������Է���3������
a g��ʱ��ɫ����Ϊ���������Է���3������
�ʴ�Ϊ������3������1���������������ͬ����ȷ��������2��Cu2O��Cu2O��Cu�Ļ������ϡ�����з�Ӧ��������ͬ��Ҳ��ȷ����
��������1�����巢��װ�����ڷ�Ӧ��ʼǰ��Ҫ���������Լ�飬C���ṩ�Ҵ�������
��2��ʵ�鿪ʼʱ���ȹر�ֹˮ��a����Һ©����������ͭ˿��λ����Ƭ�̣�������Ӧ�������������� Eװ�ü��Ⱥ�ɫͭ�仯Ϊ��ɫ����ͭ��Ȼ���ֹˮ��a���Ҵ��������������ͭ����������ȩ��
��3��������Χ�ơ���ɫ�������룬��ΪͭҲ�Ǻ�ɫ�ģ����Ժ�ɫ����������Cu��Cu2O���߶�����
����1������Cu��Cu2O������ϡ���ᷴӦ�����з�����
����2��Cu2O��ϡ���ᷴӦ��Cu���ɣ��в�����Cu����ϡ���ᷴӦ�в��������н��
����3��ͭ�ڿ�������������CuO��Cu2O�ڿ���������Ҳ��������ͭ�����������ӵ�������ͬ���ü�ֵ�����з������ȷ����ɫ�����
���������⿼�����������ʵ�ʵ�鷽������ƺͷ����жϣ���Ҫ���Ҵ��Ĵ�������Ӧ�IJ��������жϺͷ�Ӧ����Ӧ�ã���Ŀ�Ѷ��еȣ�
�ʴ�Ϊ�����װ�õ������ԣ�����ƽ�ȵ��Ҵ�������
��2��ʵ�鿪ʼʱ���ȹر�ֹˮ��a����Һ©����������ͭ˿��λ����Ƭ�̣�������Ӧ�������������� Eװ�ü��Ⱥ�ɫͭ�仯Ϊ��ɫ����ͭ��Ȼ���ֹˮ��a���Ҵ��������������ͭ����������ȩ��ͭ�ɺ�ɫ�仯Ϊ��ɫ������ֱ�Ϊ��ɫ����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2
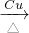 2CH3CHO+2H2O��
2CH3CHO+2H2O���ʴ�Ϊ����ڵ�ͭ˿�ֱ�죬���ֽ���仯��2CH3CH2OH+O2
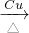 2CH3CHO+2H2O��
2CH3CHO+2H2O����3������1������Cu��Cu2O������ϡ���ᷴӦ���õ���ɫ��Һ�����Է���1���У�
����2��Cu2O��ϡ���ᷴӦ��Cu���ɣ��в�����Cu����ϡ���ᷴӦ�в��������Է���2���У�
����3��ͭ�ڿ�������������CuO��Cu2O�ڿ���������Ҳ��������ͭ�����������ӵ�������ͬ��
agCu�ڿ��������գ���ȫ��Ӧ��������Ϊ
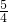 ag��agCu2O�ڿ��������գ���ȫ��Ӧ��������Ϊ
ag��agCu2O�ڿ��������գ���ȫ��Ӧ��������Ϊ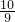 ag������ag��ɫ�����ڿ�����������ȫ��Ӧ��bΪ
ag������ag��ɫ�����ڿ�����������ȫ��Ӧ��bΪ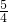 agʱ��ɫ����Cu����bΪ
agʱ��ɫ����Cu����bΪ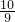 ag��ʱ��ɫ���� Cu2O����
ag��ʱ��ɫ���� Cu2O����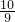 a g��b��
a g��b��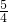 a g��ʱ��ɫ����Ϊ���������Է���3������
a g��ʱ��ɫ����Ϊ���������Է���3�������ʴ�Ϊ������3������1���������������ͬ����ȷ��������2��Cu2O��Cu2O��Cu�Ļ������ϡ�����з�Ӧ��������ͬ��Ҳ��ȷ����
��������1�����巢��װ�����ڷ�Ӧ��ʼǰ��Ҫ���������Լ�飬C���ṩ�Ҵ�������
��2��ʵ�鿪ʼʱ���ȹر�ֹˮ��a����Һ©����������ͭ˿��λ����Ƭ�̣�������Ӧ�������������� Eװ�ü��Ⱥ�ɫͭ�仯Ϊ��ɫ����ͭ��Ȼ���ֹˮ��a���Ҵ��������������ͭ����������ȩ��
��3��������Χ�ơ���ɫ�������룬��ΪͭҲ�Ǻ�ɫ�ģ����Ժ�ɫ����������Cu��Cu2O���߶�����
����1������Cu��Cu2O������ϡ���ᷴӦ�����з�����
����2��Cu2O��ϡ���ᷴӦ��Cu���ɣ��в�����Cu����ϡ���ᷴӦ�в��������н��
����3��ͭ�ڿ�������������CuO��Cu2O�ڿ���������Ҳ��������ͭ�����������ӵ�������ͬ���ü�ֵ�����з������ȷ����ɫ�����
���������⿼�����������ʵ�ʵ�鷽������ƺͷ����жϣ���Ҫ���Ҵ��Ĵ�������Ӧ�IJ��������жϺͷ�Ӧ����Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д�
�����Ŀ

 ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������
ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������