��Ŀ����
����Ŀ��̽��NaHSO3��Һ�ֱ���CuCl2��CuSO4��Һ�ķ�Ӧ��

��֪����![]() ������ɫ��Һ��
������ɫ��Һ��
��![]()
�ش��������⣺
(1)ʵ������������ɫ����ΪSO2����պ�е�ˮ�ĵ�����ֽ�ӽ��Թܿڣ��۲쵽_________________________����Ӧ�����ӷ���ʽΪ______________________��
(2)��ʵ��������SO2��ԭ����з�����������ּ��裺
��Cu2+ˮ��ʹ��Һ��c(H+)����
��Cl������ʱ��Cu2+��![]() ��Ӧ����CuCl��ɫ��������Һ��c(H+)����
��Ӧ����CuCl��ɫ��������Һ��c(H+)����
ʵ��֤��������������ʵ��֤����____________________����������ʵ������Ӧ�����ӷ���ʽ��_________________________��H++![]() ��SO2��+H2O��
��SO2��+H2O��
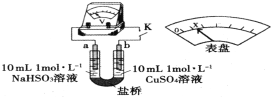
(3)ͨ������ʵ������������֪��Cl����ǿ��Cu2+�������ԡ�����ʵ��֤ʵ�˴˽��ۡ�ʵ�鷽�����պ�K����ѹ����ָ��ƫת����x��������U�ι�___________(��ȫʵ�����������)��װ���У����ŵ�������______________________________(�����������)��
(4)��ʵ��������Һ����24Сʱ����Ⱥõ���ɫ�����������飬��ɫ�����к���Cu+��Cu2+��![]() �����ʵ�飬֤����ɫ�����к���Cu+__________________��
�����ʵ�飬֤����ɫ�����к���Cu+__________________��
���𰸡���ɫ��ȥ SO2+I2+2H2O��![]() +2I��+4H+ ʵ����������c(Cu2+)��ͬ����ʵ������δ������ 2Cu2++2Cl��+
+2I��+4H+ ʵ����������c(Cu2+)��ͬ����ʵ������δ������ 2Cu2++2Cl��+![]() +H2O��2CuCl��+
+H2O��2CuCl��+![]() +3H+ �Ҳ����һ����NaCl���壬�ܽ�۲쵽��ѹ��ָ��ƫת��� �����������������Һ��ʹ���Ӷ����ƶ������ɱպϻ�·��ƽ����������� ȡϴ���ĺ�ɫ�������Թ��У��μ�����Ũ��ˮ�������ܽ⣬�õ�dz��ɫ��Һ��¶���ڿ�����һ��ʱ�����Һ��Ϊ����ɫ������֤����ɫ�����к���Cu+
+3H+ �Ҳ����һ����NaCl���壬�ܽ�۲쵽��ѹ��ָ��ƫת��� �����������������Һ��ʹ���Ӷ����ƶ������ɱպϻ�·��ƽ����������� ȡϴ���ĺ�ɫ�������Թ��У��μ�����Ũ��ˮ�������ܽ⣬�õ�dz��ɫ��Һ��¶���ڿ�����һ��ʱ�����Һ��Ϊ����ɫ������֤����ɫ�����к���Cu+
��������
��ʵ��ͨ��̽��NaHSO3�ֱ���CuCl2��CuSO4��Һ�ķ�Ӧ���֣�CuCl2��Һ������NaHSO3��Һ��Ӧ��������Ͱ�ɫ������ͨ����һ����ʵ��̽�����������ԭ��Ͱ�ɫ��������Ҫ�ɷ֣�ͨ��ʵ��ó�����Cl-�Ĵ���ʹ����Һ�в���������Ͱ�ɫ������ԭ����Cl-�Ĵ���ʹ��Cu2+����������ǿ��Cu2+�����������������Ӧ���ɴ����������ӣ�ͭ���ӱ���ԭΪ��ͭ���ӣ�����������Һ�е������������Ӧ���ɶ����������壬��ͭ��������Һ�е������ӷ�Ӧ���ɰ�ɫ�������ݴ˷�����
(1)��ʵ��������������ΪSO2��SO2�л�ԭ�ԣ����Խ�I2��ԭΪI-����Ӧ�����ӷ���ʽΪSO2+I2+2H2O��![]() +2I��+4H+�����ŷ�Ӧ�IJ��Ͻ��У���ˮ�еĵ⺬�����Ͻ��ͣ��ʷ�Ӧ����Ϊ��ɫ��ȥ��
+2I��+4H+�����ŷ�Ӧ�IJ��Ͻ��У���ˮ�еĵ⺬�����Ͻ��ͣ��ʷ�Ӧ����Ϊ��ɫ��ȥ��
(2)��������ʵ�飬����ʵ���ж�����Cu2+������ʵ������δ�����ݣ�˵��ʵ����������SO2��Cu2+��ˮ���أ������������������������ͭ���������Ӳ�ͬ��˵���������ӵIJ�ͬ����SO2�����ɣ�ʵ��������Cl-�Ĵ�����Cu2+��![]() ������Ӧ����CuCl��������ʵ��������Ӧ�����ӷ���ʽΪ2Cu2++2Cl��+
������Ӧ����CuCl��������ʵ��������Ӧ�����ӷ���ʽΪ2Cu2++2Cl��+![]() +H2O��2CuCl��+
+H2O��2CuCl��+![]() +3H+��H++
+3H+��H++![]() ��SO2��+H2O��
��SO2��+H2O��
(3)��Ҫ��֤Cl-��������Cu2+�������ԣ���������ͭһ���м���Cl-�������ܸı�Cu2+Ũ�ȣ���ֻ�ܼ�����������ι��壬ͬʱ���������Ӳ��ܸı���Һ����ԣ�����������ͭһ�����NaCl���壨��KCl���壩���������ܽ��۲��ѹ��ָ���ƫת�̶ȣ�ָ��ƫת���˵��Cl-��������Cu2+�������ԣ���װ���м������ſ��Ժܺõ����U�����˵ĵ������Һ�����ŷ�Ӧ�IJ��Ͻ���U���������ӵ�ɲ�ƽ�⣬���ſ���ƽ����������ɣ�ͬʱ�����е����ӻ����Զ����ƶ������ɱպϻ�·��
(4) ��ʵ��������Һ����24Сʱ����Ⱥõ���ɫ��������ɫ�����к���Cu+��Cu2+��![]() ����Ҫ��֤��ɫ�������Ƿ���Cu+�����Խ���ɫ��������Ũ��ˮ�У���ʱ���ں�ɫ�����к���Cu2+��ʹ��Һ���dz��ɫ���ڿ����з���һ��ʱ�����Һ�������ɫ���������ں�ɫ�����к���Cu+����Ũ��ˮ�з�����֪�����ڵķ�Ӧ��һ��ʱ���Ժ�Cu+�������е�������������Cu2+��ʹ��Һ��ɫ��������ʵ�鲽��Ϊ��ȡϴ���ĺ�ɫ�������Թ��У��μ�����Ũ��ˮ�������ܽ⣬�õ�dz��ɫ��Һ��¶���ڿ�����һ��ʱ�����Һ��Ϊ����ɫ������֤����ɫ�����к���Cu+��
����Ҫ��֤��ɫ�������Ƿ���Cu+�����Խ���ɫ��������Ũ��ˮ�У���ʱ���ں�ɫ�����к���Cu2+��ʹ��Һ���dz��ɫ���ڿ����з���һ��ʱ�����Һ�������ɫ���������ں�ɫ�����к���Cu+����Ũ��ˮ�з�����֪�����ڵķ�Ӧ��һ��ʱ���Ժ�Cu+�������е�������������Cu2+��ʹ��Һ��ɫ��������ʵ�鲽��Ϊ��ȡϴ���ĺ�ɫ�������Թ��У��μ�����Ũ��ˮ�������ܽ⣬�õ�dz��ɫ��Һ��¶���ڿ�����һ��ʱ�����Һ��Ϊ����ɫ������֤����ɫ�����к���Cu+��

����Ŀ��������Ũ�Ⱦ�Ϊ0.1mol/L ����������Һ����pH �����ʾ������˵����ȷ������ ��
��� | �� | �� | �� | �� |
��Һ | CH3COONa | NaHCO3 | Na2CO3 | NaClO |
pH | 8.8 | 9.7 | 11.6 | 10.3 |
A. ����Ũ�ȵ�CH3COOH ��HClO��Һ��pHС����HClO
B. Na2CO3��NaHCO3��Һ���������ͬ
C. ��Һˮ�ĵ���̶ȣ���>��>��>��
D. NaHCO3��Һ�У�c (Na+) =c (CO32- )+c (HCO3- )+c (H2CO3)