题目内容
14.某实验小组用下列装置进行乙醇催化氧化的实验,并检验反应的主产物.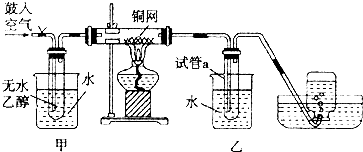
(1)实验过程中铜网出现红色和黑色交替的现象,请用化学反应方程式解释这一现象2Cu+O2△_△––––––2CuO、CH3CH2OH+CuO△→△→CH3CHO+Cu+H2O.在不断鼓入空气的情况下,熄灭酒精灯,反应仍能继续进行,说明乙醇氧化反应是放热反应(填“吸热”或“放热”).
(2)甲和乙两个水浴作用不相同.甲的作用是加热产生乙醇蒸气;乙的作用是冷凝产物.
(3)反应进行一段时间后,干燥试管a中能收集到的有机物都有CH3CHO、C2H5OH (填结构简式).该反应的主产物与新制氢氧化铜发生反应的化学方程式是:CH3CHO+2Cu(OH)2+NaOH△→△→CH3COONa+Cu2O↓+3H2O,集气瓶中收集到的气体的主要成分是N2(填化学式).
(4)若试管a中收集到的液体有刺激性气味,用紫色石蕊试纸检验,试纸显红色,说明液体中还含有CH3COOH (填结构简式),要除去该物质,可先在混合液中加入c (填写字母),然后蒸馏,得到主产物.
a.饱和氯化钠溶液 b.苯 c.饱和碳酸氢钠溶液 d.四氯化碳.
分析 (1)乙醇在铜做催化剂条件被氧气氧化成乙醛,属于加氧的氧化反应,方程式为:2CH3CH2OH+O2→铜△2CH3CHO+2H2O,熄灭酒精灯,反应仍能继续进行,说明该反应是放热反应;
(2)依据乙醇和乙醛的物理性质:二者都容易挥发.乙醇是反应物,应转化成乙醇蒸汽进入到硬质试管内参与反应;乙醛是产物,降低温度使其转化成液态,所以前者用热水浴,后者用冷水浴;
(3)根据物质的沸点高低不同来确定获得的物质,该反应的主产物为乙醛,与新制氢氧化铜发生反应生成乙酸钠、氧化亚铜和水,结合空气的成分以及发生的反应确定剩余的气体成分;
(4)酸能使紫色石蕊试纸显红色,乙酸具有酸的通性,碳酸氢钠可以和乙酸反应,其余不可.
解答 解:(1)铜丝变黑是因为发生反应:2Cu+O2△_2CuO;后来变红是因为发生反应:CH3CH2OH+CuO△→CH3CHO+Cu+H2O,该反应是乙醇的催化氧化,铜在反应中做催化剂;
熄灭酒精灯,反应仍能继续进行,说明该反应时一个放热反应,
故答案为:2Cu+O2△_2CuO、CH3CH2OH+CuO△→CH3CHO+Cu+H2O;放热;
(2)根据反应流程可知:在甲处用热水浴加热使乙醇挥发与空气中的氧气混合,有利于下一步反应;乙处作用为冷水浴,降低温度,使生成的乙醛冷凝成为液体,沉在试管的底部,
故答案为:加热产生乙醇蒸气;冷凝产物;
(3)乙醇的催化氧化实验中的物质:乙醇、乙醛的沸点高低不同,在试管a中能收集这些不同的物质,该反应的主产物为乙醛,与新制氢氧化铜发生反应CH3CHO+2Cu(OH)2+NaOH△→CH3COONa+Cu2O↓+3H2O,空气的成分主要是氮气和氧气,氧气参加反应后剩余的主要是氮气,
故答案为:乙醛、乙醇;CH3CHO+2Cu(OH)2+NaOH△→CH3COONa+Cu2O↓+3H2O;氮气;
(4)若试管a中收集到的液体用紫色石蕊试纸检验,试纸显红色,说明液体中还含有乙酸,四个选择答案中,只有碳酸氢钠可以和乙酸反应,生成乙酸钠、水和二氧化碳,实现两种互溶物质的分离用蒸馏法,然后蒸馏,得到主产物,
故答案为:乙酸;c.
点评 本题考查了乙醇的催化氧化实验,掌握化学实验基本操作以及乙醇的催化氧化反应历程是解答的关键,题目难度中等.

①过滤,
②加稍过量的NaOH溶液,
③向滤液中加适量盐酸,
④加稍过量的Na2CO3溶液,
⑤加稍过量的BaCl2溶液,
⑥将滤液蒸发结晶.
| A. | ②④⑤①③⑥ | B. | ⑤②④①③⑥ | C. | ④②⑤①③⑥ | D. | ②⑤④③①⑥ |
| A. | 苯与溴反应制溴苯 | |
| B. | 苯与浓硝酸在浓硫酸作用下制硝基苯 | |
| C. | 乙醇与乙酸的酯化反应 | |
| D. | 一定条件下苯与氢气反应制环己烷 |
| A. | 一定有乙烯 | B. | 一定有甲烷 | ||
| C. | 可能有乙烷 | D. | 一定是甲烷和乙烯的混合物 |
| A. | 燃烧 | B. | NaOH溶液 | ||
| C. | 溴的四氯化碳溶液 | D. | 酸性高锰酸钾溶液 |
| A. | 脂肪 | B. | 蛋白质 | C. | 葡萄糖 | D. | 纤维素 |
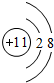 .B存在同素异形现象,其中常用作消毒剂的是O3.(填化学式)
.B存在同素异形现象,其中常用作消毒剂的是O3.(填化学式) .
.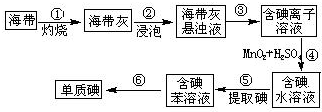 碘在工农业生产和日常生活中有重要用途.
碘在工农业生产和日常生活中有重要用途.