��Ŀ����
ij��ѧ��ȤС������ij����������ͭп����ȡ����ZnOʵ���������£�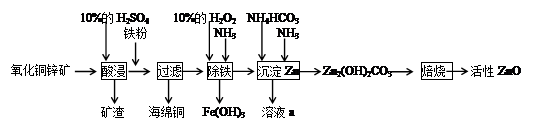
��ش��������⣺
��1���������ۺ�����Ӧ�����ӷ���ʽΪ__________��
��2���ס�����ͬѧѡ���������������ò�ͬ�ķ�������ȡ������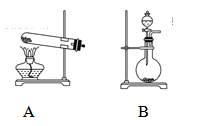
�ټ�ͬѧʹ�õ�ҩƷ����ʯ�����Ȼ�泥���Ӧѡ��װ��______����дװ�ô��ţ������ɰ����Ļ�ѧ����ʽΪ____________��
����ͬѧѡ����װ��B����ʹ�õ�����ҩƷ������Ϊ_______________��
��3��H2O2��������_____________��
��4�����������еõ���Fe(OH)3����KClO��Һ�ڼ��Ի������������õ�һ�ָ�Ч�Ķ��ˮ��������K2FeO4�����÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ____________��
��5����֪��Һa�к���CO32-��SO42-������������ӣ���ֻ����ȡ��һ����Ʒ�������������Ӵ��ڵ�ʵ���������Ϊ___________________��
��1��Fe+Cu2+= Fe2++Cu
��2��A Ũ��ˮ�ͼ�ʯ�ң�����ʯ�һ��������ƹ��壩
��3����Fe2+������Fe3+
��4��3��2
��5��ȡ������Һa���Թ��У��μ��Թ��������ᣬ����ɫ���ݲ�������˵����CO32-�������μ��Ȼ�����Һ��������ɫ������˵����SO42-
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����˵����ȷ����
| A��������������NaCl��Һ�͵��۽��� |
| B����ȥ�����������ӣ��������м���������NaOH��Һ����� |
| C����������ϡ����ɳ�ȥBaCO3������������BaSO4 |
| D����������ϡ��Һ����μ���ϡ��ˮ����ɫ����ǡ���ܽ⣬����������Һ |
���ᾧõ�塱����ǿ�ҵ�õ����������һ�ֺܺõĶ�������仯ѧ����Ϊ���������ȼ���������ͨ�������ȼ������״��ʹ�����Ϊԭ���Ʊ���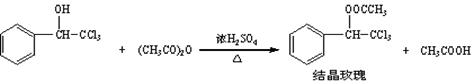
��֪��
| ���ȼ������״� | ʽ����224.5����ɫҺ�塣������ˮ�������Ҵ��� |
| ������ | ��ɫҺ�塣����ˮ�γ����ᣬ�����Ҵ��� |
| �ᾧõ�� | ʽ����267.5����ɫ����ɫ���塣�۵㣺88�档������ˮ�������Ҵ���70��ʱ���Ҵ����ܽ��Ϊa g�� |
| ���� | ��ɫ����ʪ��Һ�壬������ˮ���Ҵ��� |

�����������Ϣ���ش��������⣺
��1������ʱ��Ӧ�ȼ������ȼ������״��ʹ�������Ȼ����������Ũ���Ტ ������Ͼ��Ⱥ������˵ļ��ȷ�ʽΪ ���ˮԡ���ȡ�����ԡ���ȡ�����
��2���ֲ�Ʒ�ijɷ��ǽᾧõ��������____________________�Ļ�����������·��������ᴿ�ͼ��飬ʵ���������Ʋ���ȷ�����ڴ������ɱ������ݡ�
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ���ֲ�Ʒ�ܽ��� �У����ֲ�Ʒ���ܼ���������Ϊ1: ��ϣ���ˮԡ���ȵ�70������ܼ�ʹ�ֲ�Ʒ����ܽ� | �õ���ɫ��Һ | |
| �� | ������1������Һ___________ | | |
| �� | ���ﲽ��2���ð�ɫ���壬 | __________________ | ��ɫ�����ǽᾧõ�� |


����Ϣ��֪���Ӹ���Ũ��Һ�л�ýϴ���IJ���Ϊ ��
��4��22.45g���ȼ������״���������������ַ�Ӧ�õ��ᾧõ��22.74g���������____����������λ��Ч���֣�
��ҵ�ϳ�����ұ��п�����е�п������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�������������Zn(NO3)2��6H2O���壬�乤������Ϊ��
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
| ��ʼ������pH | 3��3 | 1��5 | 6��5 | 4��2 | 5��4 |
| ������ȫ��pH | 5��2 | 3��7 | 9��7 | 6��7 | 8��0 |
���ڡ�����������У�Ϊ���п�Ľ������ʣ���ͨ����������衱�⣬���ɲ�ȡ�Ĵ�ʩ�� ��
���������������жദ�漰�����ˡ���ʵ�����й��˲�����Ҫʹ�õIJ��������� ��
���ڡ�����I�������У����ټ�������H2O2��Һ��H2O2��Fe2+��Ӧ�����ӷ���ʽΪ ��ΪʹFe(OH)3��Al(OH)3������ȫ����Zn(OH)2��������Ӧ������Һ��pH��ΧΪ ������Fe3+�Ƿ������ȫ��ʵ������� ��
�ȼ���Zn�۵������� ��������A���������� ��


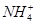 ��K����Na����Mg2����Ba2����Al3����Cl����I����
��K����Na����Mg2����Ba2����Al3����Cl����I����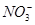 ��
��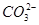 ��S2����
��S2����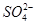 ��
��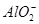 ��
��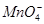 ��ȡ����Һ��������ʵ�飺
��ȡ����Һ��������ʵ�飺

