��Ŀ����
����Ŀ���״�����Ҫ�Ļ���ԭ�ϣ����úϳ�����CO��H2��CO2���ڴ����������ºϳɼ״������ܷ����ķ�Ӧ���£�
��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H1��-49.58kJ/mol K1
CH3OH(g)��H2O(g) ��H1��-49.58kJ/mol K1
��CO(g)��2H2(g)![]() CH3OH(g) ��H2��-90.77 kJ/mol K2
CH3OH(g) ��H2��-90.77 kJ/mol K2
��CO2(g)��H2(g)![]() CO(g)��H2O(g) ��H3 K3
CO(g)��H2O(g) ��H3 K3
��1����Ӧ�۵���H3��________����ѧƽ�ⳣ��K3��K1��K2�Ĵ�����ϵ��K3��_____��
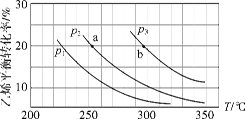
��2��Ҫʹ��Ӧ�ڵ����ʺ�ת���ʶ�������Ҫ�ı��������___________����5MPa�£�Ҫ��߷�Ӧ�ڵ�ת���ʣ��ɲ�ȡ�Ĵ�ʩ��__________��_________������������
��3������Ӧ���ں����ܱ������н��У����п����жϸ÷�Ӧ�ﵽƽ�����_______�����ţ���
A��v��(H2)��v��(CH3OH) B�������ѹǿ����
C��c(H2)��c(H2O)��ֵ���� D��������ܶȲ���
��4����һ���¶Ⱥʹ��������£���1L�ܱ������г���1molCO2��3molH2������Ӧ�١���CO2��ƽ��ת����Ϊ50%ʱ������״����������Ϊ________�����¶��£�����Ӧ��ƽ�ⳣ��K��__________�������������ٳ���0.5molH2��0.5molH2O(g)��������������ʱƽ��_______�ƶ�������������������������������
���𰸡�+41.19 kJ/mol K1/K2 ��ѹ ���� ��ʱ������״� BC 16.7% 0.148 ����
��������
�Ÿ��ݸ�˹���ɵ�һ����Ӧ��ȥ�ڶ�����Ӧ�õ���Ӧ�ۣ�����ʽ�����ƽ�ⳣ�������
�Ʒ�Ӧ���������С�ķ��ȷ�Ӧ��һ��ѹǿ�£���Ӱ��ƽ���ƶ���������߷�Ӧ�ڵ�ת���ʵ�������
��A��v��(H2)��v��(CH3OH)��һ��һ�棬�����ʱȲ����ڼ���ϵ���ȣ�����˵���ﵽƽ�⣻B����Ӧ�������С�ķ�Ӧ������Ӧ��ѹǿ��С���������ѹǿ���䣬��ﵽƽ�⣻C��c(H2)��c(H2O)��ֵ���䣬��ﵽƽ�⣻D���ܶȵ�����������������������������������䣬����������䣬������ܶ�ʼ�ղ��䣬��˲�����Ϊ�ж�ƽ���־��
�Ƚ�������ʽ���������״���������������¶��£���������Ӧ��ƽ�ⳣ���������������ٳ���0.5 mol H2��0.5 mol H2O(g)������Ũ���̺�ƽ�ⳣ���Ƚϡ�
�Ÿ��ݸ�˹���ɵ�һ����Ӧ��ȥ�ڶ�����Ӧ�õ���Ӧ������H3��49.58 kJ��mol1(90.77 kJ��mol1) = +41.19 kJ��mol1������ʽ�����ƽ�ⳣ���������˻�ѧƽ�ⳣ��K3��K1��K2�Ĵ�����ϵ��K3��![]() ���ʴ�Ϊ��+41.19 kJ��mol1��
���ʴ�Ϊ��+41.19 kJ��mol1��![]() ��
��
�Ʒ�Ӧ���������С�ķ��ȷ�Ӧ��Ҫʹ��Ӧ�������ʺ�ת���ʶ�������Ҫ�ı�������Ǽ�ѹ����5 MPa�£�����ƽ�������ƶ�����ʱ�ķ�����״���ƽ�������ƶ���������߷�Ӧ����ת���ʣ��ʴ�Ϊ����ѹ�����¡���ʱ������״���
��A��v��(H2)��v��(CH3OH)��һ��һ�棬�����ʱȲ����ڼ���ϵ���ȣ�����˵���ﵽƽ�⣬��A���������⣻
B����Ӧ�������С�ķ�Ӧ������Ӧ��ѹǿ��С���������ѹǿ���䣬��ﵽƽ�⣬��B�������⣻
C��c(H2)��c(H2O)��ֵ���䣬��ﵽƽ�⣬��C�������⣻
D���ܶȵ�����������������������������������䣬����������䣬������ܶ�ʼ�ղ��䣬��˲�����Ϊ�ж�ƽ���־����D���������⡣
������������ΪBC��
����һ���¶Ⱥʹ��������£���1 L�ܱ������г���1 mol CO2��3 mol H2������Ӧ������CO2��ƽ��ת����Ϊ50%ʱ�� ������״����������Ϊ
������״����������Ϊ![]() �����¶��£�����Ӧ��ƽ�ⳣ��
�����¶��£�����Ӧ��ƽ�ⳣ��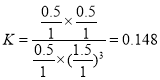 �������������ٳ���0.5 mol H2��0.5 mol H2O(g)��
�������������ٳ���0.5 mol H2��0.5 mol H2O(g)�� ��������������ʱƽ�������ƶ����ʴ�Ϊ��16.7%��0.148������
��������������ʱƽ�������ƶ����ʴ�Ϊ��16.7%��0.148������