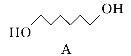
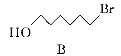
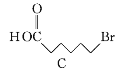
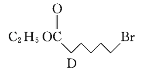
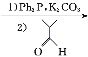
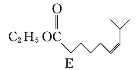
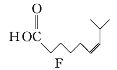
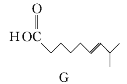
��Ŀ����
����Ŀ���ڽ�����ǽ�����������ϣ���NaBH4��������ѧ�������������Եõ���Ӳ����ʴ�ı�����(3Ni3B+Ni)����Ӧ�����ӷ���ʽΪ��
20Ni2++16BH4-+34OH-+6H2O===2(3 Ni3B+ Ni)+10B(OH)4-+35H2![]()
��1��Ni2+��̬��������Ų�ʽΪ________��
��2����BH4-��Ϊ�ȵ������һ�ַ���Ϊ______________![]() �ѧʽ
�ѧʽ![]() ��
��
��3��B(OH)4-����ԭ�ӹ�����ӻ�������________��1mol B(OH)4-���ЦҼ�����ĿΪ________mol��
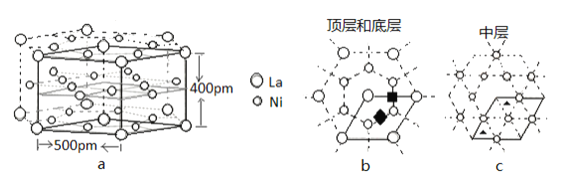
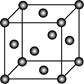
��4��Ni�ľ����ṹ��ͼ��ʾ����������ÿ����ԭ����Χ�����������ԭ����ĿΪ________��
��5��NiCl2![]() 6H2O��SOCl2�����м���ʱ������NiCl2�������������塣д���÷�Ӧ�Ļ�ѧ����ʽ��__________________��
6H2O��SOCl2�����м���ʱ������NiCl2�������������塣д���÷�Ӧ�Ļ�ѧ����ʽ��__________________��
���𰸡�1s22s22p63s23p63d8 CH4��SiH4 sp3 8 12 NiCl26H2O+6SOCl2=NiCl2+6SO2+12HCl
��������
��1������Ni��28��Ԫ�أ���д![]() ��̬��������Ų�ʽ��
��̬��������Ų�ʽ��
��2������![]() �е�������Ϊ10��ԭ������Ϊ5�� ��д�ȵ����壻
�е�������Ϊ10��ԭ������Ϊ5�� ��д�ȵ����壻
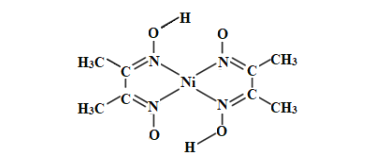
��3������![]() �еļ۲���Ӷ���Ϊ
�еļ۲���Ӷ���Ϊ![]() ���ж���ԭ�ӹ�����ӻ����ͣ�
���ж���ԭ�ӹ�����ӻ����ͣ�
��4������Ni�ľ����ṹ��֪����������ÿ����ԭ����Χ�����������ԭ����Ŀ��
��1��Ni��28��Ԫ�أ�![]() ��̬��������Ų�ʽΪ1s22s22p63s23p63d8��
��̬��������Ų�ʽΪ1s22s22p63s23p63d8��![]() ��
��
�ʴ�Ϊ��1s22s22p63s23p63d8��![]() ��
��
��2��![]() �е�������Ϊ10��ԭ������Ϊ5����
�е�������Ϊ10��ԭ������Ϊ5����![]() ԭ������ͬ����������Ҳ��ͬ��һ�ַ���ΪCH4��SiH4�ȣ��ʴ�Ϊ��CH4��SiH4��
ԭ������ͬ����������Ҳ��ͬ��һ�ַ���ΪCH4��SiH4�ȣ��ʴ�Ϊ��CH4��SiH4��
��3��![]() �еļ۲���Ӷ���Ϊ
�еļ۲���Ӷ���Ϊ![]() ������ԭ�ӹ�����ӻ�������
������ԭ�ӹ�����ӻ�������![]() ��1mol B(OH)4-����
��1mol B(OH)4-����![]() ����
����![]() �������е�
�������е�![]() ������ĿΪ8mol���ʴ�Ϊ��
������ĿΪ8mol���ʴ�Ϊ��![]() ��8��
��8��
��4������Ni�ľ����ṹ��֪����������ÿ����ԭ����Χ�����������ԭ����ĿΪ12���ʴ�Ϊ��12��
��5��NiCl2![]() 6H2O��SOCl2�����м���ʱ������NiCl2��SO2��HCl���÷�Ӧ�Ļ�ѧ����ʽ��NiCl2
6H2O��SOCl2�����м���ʱ������NiCl2��SO2��HCl���÷�Ӧ�Ļ�ѧ����ʽ��NiCl2![]() 6H2O+6 SOCl2= NiCl2+6SO2+12HCl��
6H2O+6 SOCl2= NiCl2+6SO2+12HCl��
�ʴ�Ϊ��NiCl2![]() 6H2O+6 SOCl2= NiCl2+6SO2+12HCl��
6H2O+6 SOCl2= NiCl2+6SO2+12HCl��