��Ŀ����
Ԫ��X λ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ2��Ԫ��Y��̬ԭ�ӵ�3p �������4�����ӡ�Ԫ��Z ��ԭ�����������������ڲ��3����
��1��X��Y���γɻ����ᄃ��ľ�����ͼ��ʾ��

����1�������У�X���ӵ���ĿΪ ��
�ڸû�����Ļ�ѧʽΪ �����侧���߳�Ϊ540.0pm�����ܶ�Ϊ ��X�������Y֮��ľ���Ϊ
��2����Y���⻯��(H2Y)�����У�Yԭ�ӹ�����ӻ������� ��
��3��Y ��Z ���γ�YZ42��
��YZ42���Ŀռ乹��Ϊ (����������)��
��д��һ����YZ42����Ϊ�ȵ�����ķ��ӵĻ�ѧʽ�� ��
��4��X���Ȼ����백ˮ��Ӧ���γ������[X(NH3)4]Cl2��1mol��������к��ЦҼ�����ĿΪ ��
��1��X��Y���γɻ����ᄃ��ľ�����ͼ��ʾ��

����1�������У�X���ӵ���ĿΪ ��
�ڸû�����Ļ�ѧʽΪ �����侧���߳�Ϊ540.0pm�����ܶ�Ϊ ��X�������Y֮��ľ���Ϊ
��2����Y���⻯��(H2Y)�����У�Yԭ�ӹ�����ӻ������� ��
��3��Y ��Z ���γ�YZ42��
��YZ42���Ŀռ乹��Ϊ (����������)��
��д��һ����YZ42����Ϊ�ȵ�����ķ��ӵĻ�ѧʽ�� ��
��4��X���Ȼ����백ˮ��Ӧ���γ������[X(NH3)4]Cl2��1mol��������к��ЦҼ�����ĿΪ ��
��1����4 ��ZnS ��4.1g/cm3��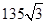 ��2��sp3
��2��sp3
��3������������ ��CCl4��SiCl4�� ��4��16NA��16��6. 02��1023��
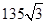 ��2��sp3
��2��sp3��3������������ ��CCl4��SiCl4�� ��4��16NA��16��6. 02��1023��
���������Ԫ��X λ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ2������X��Zn��Ԫ��Y��̬ԭ�ӵ�3p �������4�����ӣ���YӦ����SԪ�ء�Ԫ��Z ��ԭ�����������������ڲ��3������Z����Ԫ�ء�
��1���ٸ��ݾ����ṹ�����ݾ�̯����֪����1�������У�X���ӵ���ĿΪ
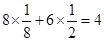 ����
������ͬ�������֪������Y����ĿҲ4�������Ըû�����Ļ�ѧʽΪZnS�����ݾ����Ľṹ��֪�����侧���߳�Ϊ540.0pm�����ܶȣ�
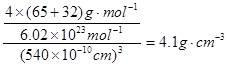 �����ݾ����Ľṹ��֪��Sԭ����Χ��4��пԭ�ӹ������������ͽṹ����X�������Y֮��ľ���Ϊ
�����ݾ����Ľṹ��֪��Sԭ����Χ��4��пԭ�ӹ������������ͽṹ����X�������Y֮��ľ���Ϊ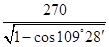 ��
��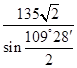 ��
��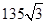 ��
����2��H2S��V�νṹ����Sԭ����sp3�ӻ���
��3������SO42��������ԭ��Sԭ�Ӻ��еŶԵ��Ӷ�������6��2��4��2����2��0������YZ42���Ŀռ乹��Ϊ�������塣
��ԭ�����ͼ۵������ֱ���ȵ��ǵȵ����壬���SO42����Ϊ�ȵ�����Ŀ�����CCl4��SiCl4�ȡ�
��4��X���Ȼ����백ˮ��Ӧ���γ������[X(NH3)4]Cl2������1�����������к���3���Ҽ����������γ��γ�4����λ�������еĦҼ���Ŀ��3��4��4��16������1mol��������к��ЦҼ�����ĿΪ16NA��16��6. 02��1023��
�������������е��Ѷ�����Ŀ��飬Ҳ�Ǹ߿��еij������͡������ۺ���ǿ�������߿������ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����Ӧ�������������������Լ��ռ����������������������ѧ����ѧ�����������ѧ����Ӧ��������������ѵ��Ǿ�����йؼ��㣬�����������պþ�̯����

��ϰ��ϵ�д�
�����Ŀ



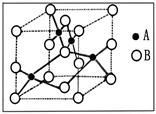
 )��
)��