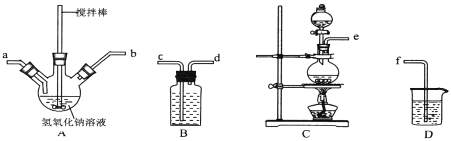
��Ŀ����
����Ŀ����������ʵ���ó�����Ӧ������ȷ����
ʵ����ʵ | ���� | |
A | ����ͬ�¶��£���1 mL0.2 mol/LNaOH��Һ�е���2��0.1 mol/LMgCl2��Һ��������ɫ�������ٵμ�2��0.1 mol/LFeCl3��Һ�������ɺ��ɫ���� | �ܽ�ȣ�Mg(OH)2>Fe(OH)3 |
B | ij������ʹʪ�����ɫʯ����ֽ��� | ������ˮ��Һһ���Լ��� |
C | ͬ��ͬѹ�£������pH=3��HA��HB������ֱ���������п��Ӧ����ˮ���ռ����壬HA�ų����������ҷ�Ӧ���ʿ� | HB�����Ա�HAǿ |
D | SiO2����������ᷴӦ������Ӧ | SiO2������������ |
A.AB.BC.CD.D
���𰸡�C
��������
A. ������ӦMgCl2+2NaOH=Mg(OH)2��+2NaCl������NaOH����������ٵ���FeCl3��Һ���ᷢ����Ӧ��FeCl3+3NaOH=Fe(OH)3��+3NaCl�����ܱȽ�Mg(OH)2��Fe(OH)3�ܽ�ȴ�С��A����
B. ij������ʹʪ�����ɫʯ����ֽ��죬�������Ϊ�������壬��ˮ��Һ�����ԣ�B����
C. HA�ų����������ҷ�Ӧ���ʿ죬HAŨ�ȱ�HB���ڷ�Ӧ������HA��Һ��c(H+)�Ƚϴ�֤��HA��Һ�д��ڵ���ƽ��HA![]() H++A-��HA�����ᣬ������HB>HA��C��ȷ��
H++A-��HA�����ᣬ������HB>HA��C��ȷ��
D. SiO2������ᷴӦ����SiF4��H2O��SiF4�����Σ����SiO2�������������D����
�ʺ���ѡ����C��

 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�