��Ŀ����
15������CH4��C2H4��C2H6��C3H6�����л��ͬ���������������У�����ͬ״�������������CH4��
ͬ����������������ȫȼ��ʱ��ȥO2����������CH4��
ͬ״����ͬ�������������������ȫȼ��ʱ��ȥO2����������C3H6��
ͬ������������������ȼ��ʱ������CO2������C2H4��C3H6������ˮ������CH4��
��120�桢1.01��105 Pa״̬�£���������̬����������������ϵ�ȼ����ͬ�����²�÷�Ӧǰ���������û�з����仯��������������CH4��C2H4��
���� ����n=$\frac{m}{M}$��֪����ͬ��������£�Ħ������ԽС�����ʵ���Խ����ͬ�����£����֮�ȵ������ʵ���֮�ȣ�
��ͬ��������£���Ԫ����������Խ����������Խ�ࣻ
ͬ״��ͬ���ʱ����������ʵ�����ȣ�1mol���ĺ�����Ϊ��x+$\frac{y}{4}$��mol���ݴ˼����жϣ�
��ͬ����ʱ��CԪ����������Խ�����ɶ�����̼Խ�࣬HԪ����������Խ������ˮ����Խ�ࣻ
���¶ȴ���100��ʱ��ˮΪ��̬����y=4ʱ��ȼ��ǰ��������䣮
��� �⣺CH4��C2H4��C2H6��C3H6��Ħ����������������n=$\frac{m}{M}$��֪����ͬ��������£�Ħ������ԽС�����ʵ���Խ����ͬ�����£����֮�ȵ������ʵ���֮�ȣ��ʼ����������
��ͬ��������£���Ԫ����������Խ����������Խ�࣬CH4��C2H4��C2H6��C3H6������C��Hԭ����Ŀ֮�ȷֱ�Ϊ��1��4��1��2��1��3��1��2���ʼ�������Ԫ�����������������������ࣻ
ͬ״��ͬ���ʱ����������ʵ�����ȣ�1mol���ĺ�����Ϊ��x+$\frac{y}{4}$��mol��1molCH4��C2H4��C2H6��C3H6�������ֱ�Ϊ2mol��3mol��3.5mol��4.5mol����C3H6��������ࣻ
CH4��C2H4��C2H6��C3H6������C��Hԭ����Ŀ֮�ȷֱ�Ϊ1��4��1��2��1��3��1��2����C2H4��C3H6̼Ԫ�������������������Ԫ���������������ͬ����ʱ��CԪ����������Խ�����ɶ�����̼Խ�࣬HԪ����������Խ������ˮ����Խ�࣬��C2H4��C3H6���ɶ�����̼��࣬��������ˮ��ࣻ
���¶ȴ���100��ʱ��ˮΪ��̬����y=4ʱ��ȼ��ǰ��������䣬����ͬ�����²�÷�Ӧǰ���������û�з����仯����CH4��C2H4��
�ʴ�Ϊ��CH4��CH4��C3H6��C2H4��C3H6��CH4��CH4��C2H4��
���� ���⿼����ȼ���йؼ������⣬�Ѷ��еȣ�ע�����ȼ��ͨʽ�жϺ��������⣬ע��Թ��ɵĹ����ܽᣮ

| A�� | �����ʯ��ˮ��ŨH2SO4 | B�� | ŨH2SO4����ˮ | ||
| C�� | ��ˮ��ŨH2SO4 | D�� | ŨH2SO4������KMnO4��Һ |
| ѡ�� | ���� | ���� | ���� |
| A | KIO3��Һ�еμ�HI���ٵμӵ�����Һ | ��Һ������ɫ | KIO3�����Ա�I2ǿ |
| B | ��Na2S��Һ�еμ����� | �������� | Cl�ķǽ����Ա�Sǿ |
| C | ��һС��Na�����Ҵ��� | �������� | �Ҵ������ǻ� |
| D | �ر�����ˮ�м�ŨH2SO4������ | �ձ���ڷ��� | Ũ��������ˮ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ԭ��������d��c��b��a | B�� | ԭ�Ӱ뾶��r ��A����r ��B����r ��D����r ��C�� | ||
| C�� | ���ʵĻ�ԭ�ԣ�A��B��D��C | D�� | ���Ӱ뾶��r ��C3-����r ��D-����r ��B+����r ��A2+�� |
| A�� | ��������NaOH��Һ��Ӧ�����Ҵ����� | |
| B�� | ��ͪ��CH3COCH3�������е������������е�����ױ�±ԭ��ȡ�� | |
| C�� | ��ϩ�ɷ����ӳɷ�Ӧ�������鲻�� | |
| D�� | ������ʵ����ĸ��ͺ��Ҵ��ֱ������������Ʒ�Ӧ�����Ͳ�����H2�� |
 ����ʮ���Աʹ�õ�����ƿ���Ծ���������Ϊԭ�ϣ��ס��ҡ������������Ǻϳɾ��������ֵĻ���ԭ�ϣ�����˵��������ǣ�������
����ʮ���Աʹ�õ�����ƿ���Ծ���������Ϊԭ�ϣ��ס��ҡ������������Ǻϳɾ��������ֵĻ���ԭ�ϣ�����˵��������ǣ�������| A�� | ��������һ�������¿��������л��߷��ӻ����� | |
| B�� | 1mol�����ʿ���2mol����ȫ��Ӧ����1mol���� | |
| C�� | �ס������ʶ���ʹ������Ȼ�̼��Һ��ɫ | |
| D�� | ��������������ˮ�����֮һ���һ�Ϊͬϵ�� |
| ��� | �� | �� | �� |
| �� | CO2 | SO2 | ʯ��ˮ |
| �� | HCl | CO2 | ʯ��ˮ |
| �� | CO2 | NH3 | Ca��Cl��2 |
| �� | NO2 | SO2 | BaCl2 |
| �� | CO2 | SO2 | Ba��NO3��2 |
| A�� | �ڢ� | B�� | �٢ڢܢ� | C�� | �٢ۢܢ� | D�� | �٢ڢۢ� |
 ��ͼ�Ƿ�������������������Ҵ�������ʵ���������ͼ��ʵ������У����漰�����η�������ֱ��ǣ�������
��ͼ�Ƿ�������������������Ҵ�������ʵ���������ͼ��ʵ������У����漰�����η�������ֱ��ǣ�������| A�� | ������ �ڹ��� �۷�Һ | B�� | �ٷ�Һ ������ �۹��� | ||
| C�� | ������ �ڷ�Һ �۷�Һ | D�� | �ٷ�Һ ������ ������ |
 ���磺
���磺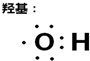
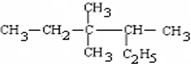 ������3��3��4-��������
������3��3��4-��������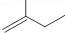 ������2-��-1-��ϩ��
������2-��-1-��ϩ��