��Ŀ����
��֪25��ʱ�й�����ĵ���ƽ�ⳣ����
�������й�˵����ȷ���ǣ� ��
A�������ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH��NaCN����pH��Na2CO3����pH��CH3COONa��
B����a mol��L��1 HCN��Һ��a mol��L��1 NaOH��Һ�������ϣ����Һ�У�c(OH�C)��c(H+)��c(Na+)��c(CN�C)
C������������μ�ˮ����Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С
D��NaHCO3��Na2CO3���Һ�У�һ����c(Na+)+ c(H+)=c(OH�C)+ c(HCO3�C) +c(CO32�C)
| ���ữѧʽ | CH3COOH | HCN | H2CO3 |
| ����ƽ�ⳣ����25�棩 | 1.8��l0�C5 | 4.9��l0�C10 | K1=4.3��l0�C7 K2=5.6��l0�C11 |
A�������ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH��NaCN����pH��Na2CO3����pH��CH3COONa��
B����a mol��L��1 HCN��Һ��a mol��L��1 NaOH��Һ�������ϣ����Һ�У�c(OH�C)��c(H+)��c(Na+)��c(CN�C)
C������������μ�ˮ����Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С
D��NaHCO3��Na2CO3���Һ�У�һ����c(Na+)+ c(H+)=c(OH�C)+ c(HCO3�C) +c(CO32�C)
B
���ݵ���ƽ�ⳣ����֪�����Ǵ��̼�HCN��HCO3����������Խ������Ӧ������Խ����ˮ�⣬����Խǿ��pHԽ��A����ȷ��B�ж���ǡ�÷�Ӧ�����ɵ�NaCNˮ�⣬�����Լ��ԣ�B��ȷ��ϡ���Ǵٽ�����ģ���˵���̶�һֱ������ģ�pH���ȼ�С����������C����ȷ��ѡ��D�в����ϵ���غ㣬����ȷ����ѡB��

��ϰ��ϵ�д�
�����Ŀ
 ��
��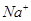 ��
��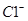 ��
��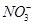
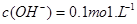 ����Һ��
����Һ��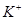 ��
��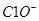 ��
��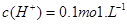 ����Һ��
����Һ��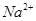 ��
��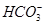 ��
��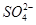
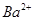 ��
��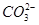 ��
��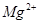 ��
��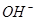 ��
��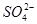
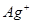 ��
��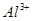 ��
��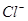
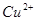 ��
��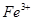 ��
��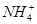 ��
��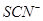
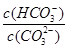 ��С
��С