��Ŀ����
11����1��CCl4������ˮ������ɫҺ�壬�밴����Ҫ����ʵ�鷽������ֻ������һ���Լ�������֧�Թֱܷ�ȡ��������CCl4������ˮ��Ȼ��ֱ���������ĵ��ʵ⣬�����Ϻ�ɫ��Һ����CCl4�����ػ�ɫ��Һ��������ˮ����2��NaCl��Һ�к���������CaCl2��ijѧ���ù�����Na2CO3ʹCa2+ת��Ϊ��������ȥ��ȷ��Ca2+�Ѿ�������ȫ��ʵ�鷽����ȡ�������ú���ϲ���Һ���Թ��У��ټ���Na2CO3��Һ����������������֤����������ȫ��
��3��ѡ������ʵ�鷽���������ʣ������뷽����������ں����ϣ�
A����ȡ��Һ�� B�����ȷֽ�C���ᾧ��D����Һ��E������F�����˷�
��D����ˮ�ͱ��Ļ���
��E�������Ȼ�̼���е�Ϊ76.75��C���ͼױ����е�Ϊ110.6��C������֪���Ȼ�̼�ͼױ����ܣ�����Һ©���Ƿ�©ˮ�ķ����ǣ��رշ�Һ©���Ļ����������м���������ˮ������©���۲��Ƿ�©ˮ������©����������ת180�Ⱥ��ٵ��ù۲죬�����Dz�©ˮ����������ܷ����ܺϸ�
���� ��1����ˮΪ�ػ�ɫ��������Ȼ�̼��ҺΪ�Ϻ�ɫ��
��2��ȷ��Ca2+�Ѿ�������ȫ��������Һ�м�����̼���Ʋ����ɳ�����
��3����ˮ�ͱ��Ļ����ֲ㣻
�����Ȼ�̼�ͼױ����ܣ����е㲻ͬ����Һ©��Ӧ������������λ���Ƿ�©ˮ��
��� �⣺��1������֧�Թֱܷ�ȡ��������CCl4������ˮ��Ȼ��ֱ���������ĵ��ʵ⣬�����Ϻ�ɫ��Һ����CCl4�����ػ�ɫ��Һ��������ˮ���ʴ�Ϊ��CCl4������ˮ��
��2��ȷ��Ca2+�Ѿ�������ȫ��ʵ�鷽����ȡ�������ú���ϲ���Һ���Թ��У��ټ���Na2CO3��Һ����������������֤����������ȫ��
�ʴ�Ϊ��ȡ�������ú���ϲ���Һ���Թ��У��ټ���Na2CO3��Һ����������������֤����������ȫ��
��3����ˮ�ͱ��Ļ����ֲ㣬�����÷�Һ�����룬�ʴ�Ϊ��D��
�����Ȼ�̼�ͼױ����ܣ����е㲻ͬ����ѡ�������룬�����Һ©���Ƿ�©ˮ�ķ���Ϊ�رշ�Һ©���Ļ����������м���������ˮ������©���۲��Ƿ�©ˮ������©����������ת180�Ⱥ��ٵ��ù۲죬�����Dz�©ˮ����������ܷ����ܺϸ�
�ʴ�Ϊ��E���رշ�Һ©���Ļ����������м���������ˮ������©���۲��Ƿ�©ˮ������©����������ת180�Ⱥ��ٵ��ù۲죬�����Dz�©ˮ����������ܷ����ܺϸ�
���� ���⿼������ļ���ͼ����������ᴿ��Ϊ��Ƶ���㣬�����ڿ���ѧ������������ʵ��������ע�����ʵ����ʡ����ʲ��켰���Ӽ��鷽������Ŀ�ѶȲ���

 �������Ƕ����ܼ����������л��ϳɷ�Ӧ�ܼ���ijʵ��С����������װ�ã��гֺͼ���װ�þ�ʡ�ԣ��ϳ������ѣ������ķ�ӦΪ��
�������Ƕ����ܼ����������л��ϳɷ�Ӧ�ܼ���ijʵ��С����������װ�ã��гֺͼ���װ�þ�ʡ�ԣ��ϳ������ѣ������ķ�ӦΪ��2CH3CH2CH2CH2OH$\stackrel{H_{2}SO_{4}}{��}$CH3CH2CH2CH2OCH2CH2CH2CH3+H2O
��Ӧ��Ͳ������������б����£�
| ������ ���� | �ܶȣ�g/mL �� | �� �㣨�棩 | �� �㣨�棩 | ˮ���ܽ��� |
| ������ | 0.810 | -89.8 | 118.0 | �� |
| ������ | 0.7689 | -95.3 | 142 | ������ˮ |
| ��ע�����������ڱ����Ȼ�����Һ�� | ||||
����ӦҺ��ȴ�����º���ʢ��25mLˮ�ķ�Һ©���У��������롢ϴ�Ӻ��ٷ����ᴿ�ɵ�������3.4g���ش��������⣺
��1����ʵ��ʱ���������ܵĽ�ˮ��Ϊ2����1��2����
��2���ڸ�ʵ���У�������ƿ���ݻ����ʺϵ���A��������ȷѡ��ǰ����ĸ����
A��50mL���������� B��150mL�������� C��250mL�������� D��500mL
��3����ʵ�������ײ����������Ļ�ѧ��Ӧ����ʽΪ��HOCH2CHBrCH2CH3 $��_{��}^{ŨH_{2}SO_{4}}$CH2=CHCH2CH3+H2O��
��4����ӦҺ��ȴ�����º���ʢ��25mLˮ�ķ�Һ©���У����ã��õ��л���IJ��������ǽ���Һ©�����ϵIJ��������ٽ���Һ©���������š����ʹ�²�Һ���������ձ������£��رշ�Һ©�������ϲ��л���ӷ�Һ©���Ͽڵ�����
��5���л���ֲ���������12mLˮ��8mL5%����������Һ��8mLˮ��8mL�����Ȼ�����Һϴ�ӣ�������������Һ��Һϴ�ӵ�Ŀ���dz�ȥ��Ʒ�е�������Ȼ�����Һϴ�ӵ�Ŀ���dz�ȥ�������������ƣ����ܼ��ٲ������ʧ��
��6��ϴ����ɺ�ͨ�����²������롢�ᴿ�����ȷ�IJ���˳����cba������ĸ����
a������ b�����ˡ� c��������ˮCaCl2
��7����ʵ�����õ��������Ѳ���Ϊ35.34%��
| A�� | ������Һ�е�c��Na+����� | |
| B�� | �ֱ��ˮϡ�͵�100 mLʱ��������Һ��pH��Ȼ��� | |
| C�� | ����Һ����ˮ�������c��OH-��֮��Ϊ10-9/10-5 | |
| D�� | �ֱ���ͬŨ�ȵ����ᷴӦ��pH=7ʱ���ĵ����������� |
| A�� | v��CH3OH��=0.3 mol•��L•min��-1 | B�� | v��O2��=0.4 mol•��L•min��-1 | ||
| C�� | v��NaOH��=0.5 mol•��L•min��-1 | D�� | v��Na2CO3��=0.01 mol•��L•s��-1 |
| A�� | 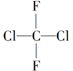 �� ��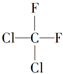 �����ֲ�ͬ������ �����ֲ�ͬ������ | |
| B�� | ������ϩ�ĵ���ΪCH2�TCHCl | |
| C�� | �������ϩ����ʹ����KMnO4��Һ��ɫ | |
| D�� | 1 mol�����������Ҵ���Ũ������¼��ȣ�������1 mol�������� |
| A�� | 200ml 2mol/L Mg Cl2��Һ | B�� | 1000 ml 2.5 mol/LNaCl | ||
| C�� | 300 ml 5 mol/L K Cl ��Һ | D�� | 250 ml 1 mol/L AlCl3 |
| A�� | ��Է���������ͬ���ṹ��ͬ�Ļ�����һ����Ϊͬ���칹�� | |
| B�� | �ṹ�ԳƵ���������һ�ȴ���ض�ֻ��һ�� | |
| C�� | ��Ϊͬ���칹��Ļ����ﲻ���ܾ�����ͬ�Ľṹ | |
| D�� | ͨʽΪCnH2n��̼ԭ������ͬ���л���һ����Ϊͬϵ�� |
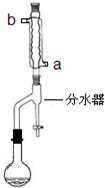 ��ƻ�����㽶��ˮ���Ĺ����д�����������������ij��ѧ������ȤС�����������������Ϊԭ�Ϻϳ�������������
��ƻ�����㽶��ˮ���Ĺ����д�����������������ij��ѧ������ȤС�����������������Ϊԭ�Ϻϳ�������������