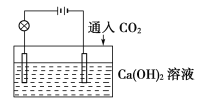
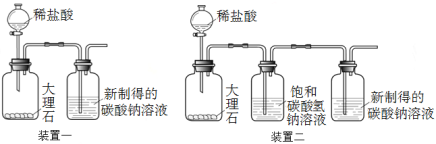
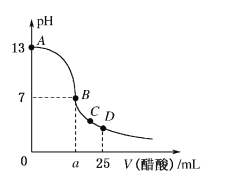
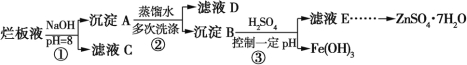
题目内容
【题目】听下面一段对话,完成五道小题,每小题仅填写一个词。这段对话你将听两遍。
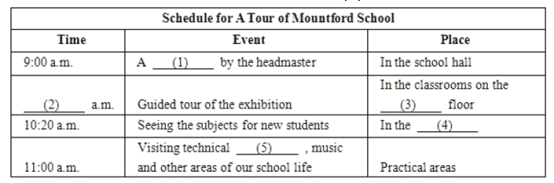
【答案】
(1) welcome speech
(2) 9:30
(3) second
(4) science labs
(5) workshops
【解析】听力原文:
Welcome to Mountford school. Thank you for choosing our school and for joining the happy Mountford family which has been educating boys since 1916. We are so happy that you've taken time off to be with us today. It is with great pleasure that we have prepared some events that we hope will please you. At 9:00 a.m., our headmaster will give a welcome speech. This will be in the school hall. Please be seated by 8:45 a.m.. Following the speech is the guided tour of the exhibition at 9:30. Here, you can see the proud history of our school, and our achievements in the field of education. The exhibition is laid out in the classrooms on the second floor. Then the guided tour of science labs at 10:20 a.m., here you can see the subjects that new students will be studying. You will also notice that our labs have excellent equipment. At 11:00 a.m., you will be taken on the tour to practical areas. This covers our technical workshops, music and other areas of our school life. At Mountford, we believe in all round development of our students. Lunch will be at 12:00. It has been specially prepared for our guests. All our teachers and student leaders will be present to answer any questions that you have in your minds. We are so happy that you could be with us today.

 阅读快车系列答案
阅读快车系列答案