��Ŀ����
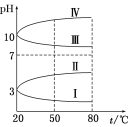
����Ŀ��10 ��ʱ����NaHCO3������Һ����ø���Һ��pH�������±仯��
�¶�(��) | 10 | 20 | 30 | ������к���ȴ��50 �� |
pH | 8.3 | 8.4 | 8.5 | 8.8 |
��ͬѧ��Ϊ������ҺpH���ߵ�ԭ����HCO![]() ��ˮ��̶���������ǿ��
��ˮ��̶���������ǿ��
��ͬѧ��Ϊ������ҺpH���ߵ�ԭ����NaHCO3���ȷֽ⣬������Na2CO3�����ƶ�Na2CO3��ˮ��̶�________NaHCO3��ˮ��̶�(����ڡ���С�ڡ�)��
��ͬѧ��Ϊ�ס��ҵ��ж϶�����֡�
������
(1)ֻҪ�ڼ�����е���Һ�м����������Լ�X����������������________(��ס����ҡ�)���ж���ȷ���Լ�X��________��
A��Ba(OH)2��Һ B��BaCl2��Һ
C��NaOH��Һ D������ʯ��ˮ
(2)��������к����Һ��ȴ��10 �棬����Һ��pH________8.3(����ڡ��������ڡ����ڡ�)����________(��ס����ҡ�)�ж���ȷ��
(3)�������ϣ�����NaHCO3�ķֽ��¶�Ϊ150 �棬������________(��ס����ҡ�)�ж��Ǵ���ģ�������______________________��
(4)����NaHCO3����ˮ��Һ�ı�����ȷ����_______________________��
a��c(Na��)��c(HCO![]() )��c(CO
)��c(CO![]() )��c(H2CO3)
)��c(H2CO3)
b��c(Na��)��c(H��)��c(HCO![]() )��c(CO
)��c(CO![]() )��c(OH��)
)��c(OH��)
c��HCO![]() �ĵ���̶ȴ���HCO
�ĵ���̶ȴ���HCO![]() ��ˮ��̶�
��ˮ��̶�
���𰸡����� (1)�� B (2)���� �� (3)�� ��ѹ�¼���NaHCO3��ˮ��Һ����Һ���¶ȴﲻ��150 �� (4)a
��������NaHCO3��Һ�У��������б仯��NaHCO3===Na����HCO![]() ��HCO
��HCO![]() ��H2O
��H2O![]() H2CO3��OH����HCO
H2CO3��OH����HCO![]()
![]() CO
CO![]() ��H����H2O
��H����H2O![]() H����OH����
H����OH����
����Ŀ�ṩ����Ϣ���Կ�����NaHCO3��Һ�ʼ��Ե�ԭ����HCO![]() ��ˮ��̶ȴ��������̶ȡ����������غ��c(Na��)��c(HCO
��ˮ��̶ȴ��������̶ȡ����������غ��c(Na��)��c(HCO![]() )��c(CO
)��c(CO![]() )��c(H2CO3)�����ݵ���غ�(��Һ�ʵ�����)��c(Na��)��c(H��)��c(HCO
)��c(H2CO3)�����ݵ���غ�(��Һ�ʵ�����)��c(Na��)��c(H��)��c(HCO![]() )��2c(CO
)��2c(CO![]() )��c(OH��)��
)��c(OH��)��