��Ŀ����
����Ŀ��������ͬѧ�քe�Ժ�+4����Ԫ�ص��������ʽ�����̽����
��1��������ͼװ�ý���ʵ�飨�������Ѽ��飬���Ⱥͼг�װ�ü���ȥ����ʵ�����һ��ʱ���C��D�ж��������Եİ�ɫ�������������ΪBaSO4
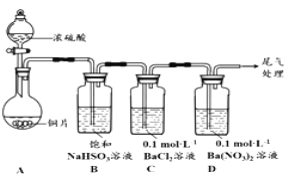
��A�з�Ӧ�Ļ�ѧ����ʽ��______________________��
��Ϊ̽��SO2��D����������Ӧ����һ��ʵ�鷢�֣����ְ�ɫ�����Ĺ����У�D��Һ��NO3-Ũ�ȼ������䡣�ݴ˵ó����ۣ�D�г��ְ�ɫ��������Ҫԭ����__________________��
��2����������ʵ��Ժ�+4����Ԫ�ص��������ʽ���̽����
��� | ʵ����� | ʵ������ |
1 | ȡ0.3g����Na2SO3���壬�����м���10mL 2mol/L���ᣬ�ٵ���4��BaCl2��Һ | ������ɫ���ݣ�����BaCl2��Һ��ʼ������4min����Һ����� |
2 | ȡ0.3g����Na2SO3���壬�����м���10mL 2mol/L HNO3���ٵ���4��BaCl2��Һ | ������ɫ���ݣ�����BaCl2��Һ��ʼ������2h����Һ����� |
3 | ȡ0.3g����Na2SO3���壬�����м���10mLŨHNO3���ٵ���4��BaCl2��Һ | ��������ɫ���壻����BaCl2��Һ����Һ�����������ذ�ɫ���� |
�������ӷ���ʽ����ʵ��1�в��������ԭ��____________________��
����ʵ��1��2��3�Աȣ����Եõ����ۣ�____________________��
����ͨ���������Ϸ��֣�Na+��ʵ��1��2�г��ֻ��ǵ�ʱ����Ӱ�죬���ǽ�һ��̽��Cl-��NO3-�����Ӱ�죺
��� | ʵ����� | ʵ������ |
4 | ȡ_______������������м���10mL 2mol/L HNO3���ٵ���4��BaCl2��Һ | ������ɫ���ݣ�����BaCl2��Һ��ʼ������20min����Һ����� |
i.ʵ��2��4�Աȣ��һ�����ۣ�Cl-�Ĵ��ڿ��Լӿ���Һ��+4����Ԫ�ص�������
iiʵ��1��4�Աȣ��һ�����ۣ�_____________________��
��ͨ������ʵ�飬��ͬѧ��Ϊ��ȷ��ij��Һ�к���SO42-��ʵ�鷽����ȡ����Һ���������ȵμ�_________������ĸ��ţ�
a. 2mol/L���ᣬ�ٵμ�BaCl2��Һ��һ��ʱ�����ְ�ɫ����
b. 2mol/L���ᣬ�ٵμ�BaCl2��Һ���������ְ�ɫ����
c. 2mol/L���ᣬ�ٵμ�BaCl2��Һ��һ��ʱ�����ְ�ɫ����
d. 2mol/L���ᣬ�ٵμ�BaCl2��Һ���������ְ�ɫ����
���𰸡�Cu+2H2SO4(Ũ)![]() CuSO4+ SO2��+2H2O (�����Ǽ��ȣ����������£���+4����Ԫ������(SO2��H2SO3)��O2��������SO42-2H++SO32-=SO2+H2O�� SO2+O2+2Ba2++2H2O=2BaSO4��+4H+��+4����Ԫ�����ʿɱ�O2��ŨHNO3����0.3g ����Na2SO3��1.17gNaClNO3-�Ĵ��ڿ��Լ�����Һ��+4����Ԫ�ص�����bd
CuSO4+ SO2��+2H2O (�����Ǽ��ȣ����������£���+4����Ԫ������(SO2��H2SO3)��O2��������SO42-2H++SO32-=SO2+H2O�� SO2+O2+2Ba2++2H2O=2BaSO4��+4H+��+4����Ԫ�����ʿɱ�O2��ŨHNO3����0.3g ����Na2SO3��1.17gNaClNO3-�Ĵ��ڿ��Լ�����Һ��+4����Ԫ�ص�����bd
��������
(1)��ͭ��Ũ������ȷ�����Ӧ��������ͭ�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O���ʴ�Ϊ��Cu+2H2SO4(Ũ)
CuSO4+SO2��+2H2O���ʴ�Ϊ��Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O����Ϊ̽��SO2��D���������ķ�Ӧ����һ��ʵ�鷢�֣����ְ�ɫ�����Ĺ����У�D��Һ��NO3-Ũ�ȼ������䣮�ݴ˵ó����ۣ�D�г��ְ�ɫ��������Ҫԭ����������Һ�ж�������ᱻ���������������ᣬ��ϱ����ӣ�Ҳ���������ᱵ�������ʴ�Ϊ�����������£���+4����Ԫ������(SO2��H2SO3)��O2��������SO42-��(2)��ȡ0.3g ����Na2SO3���壬�����м���10mL 2molL-1 ���ᣬ�ٵ���4��BaCl2��Һ��������ɫ����Ϊ�����������壬����BaCl2��Һ��ʼ������4min����Һ����ǣ�˵�����������������������������ᣬ��ϱ������������ᱵ��ɫ��������Ӧ�����ӷ���ʽΪ��2H++SO32-�TSO2+H2O��2SO2+O2+2Ba2++2H2O�T2BaSO4��+4H+��2H2SO3+O2+2Ba2+�T2BaSO4��+4H+���ʴ�Ϊ��2H++SO32-�TSO2+H2O��2SO2+O2+2Ba2++2H2O�T2BaSO4��+4H+��2H2SO3+O2+2Ba2+�T2BaSO4��+4H+������ʵ��1˵������������Ҳ��������+4����Ԫ�صĻ����ʵ��2˵��������Һ����������Ӷ�������Ӧ�������ã����ֳ���ʱ�䳤��ʵ��3��Ũ����������+4����Ԫ�ػ�����������������ӣ����ֳ����죬�Աȿ�֪������Ũ���ᶼ���������������ʴ�Ϊ����+4����Ԫ�����ʿɱ�O2��ŨHNO3��������̽��Cl-��NO3-�����Ӱ�죬i��ʵ��2��4�Աȣ��һ�����ۣ�Cl-�Ĵ��ڿ��Լӿ���Һ��+4����Ԫ�ص�������ʵ��4����Ҫ�ṩ��ʵ��1����ͬ��������ʵ��̽��������Ҫ0.01L��2mol/L=0.02mol���Ȼ��Ƶ�����=0.02mol��58.5g/mol=1.17g���Ա�ʵ��1�жϳ��ֳ�����ʱ�������ȡ0.3g ����Na2SO3��1.17gNaCl���壬�����м���10mL 2molL-1 HNO3���ٵ���4��BaCl2��Һ���۲���ֳ�����ʱ�䣬�ʴ�Ϊ��0.3g����Na2SO3��1.17gNaCl��ii��ʵ��1��4�Աȣ���ͬ������������ᣬ��������ͬ�����ֳ�����ʱ����������Һ�п죬�һ����������������Ӽ���+4����Ļ������������ʵ��1��4�Աȣ��һ�������ǣ�NO3-�Ĵ��ڿ��Լ�����Һ��+4����Ԫ�ص��������ʴ�Ϊ��NO3-�Ĵ��ڿ��Լ�����Һ��+4����Ԫ�ص��������ܶԱ�����ʵ��ȷ��ij��Һ�к���SO42-��ʵ�鷽���ǣ�ʵ��1��֪��ȡ0.3g ����Na2SO3���壬�����м���10mL 2molL-1 ���ᣬ�ٵ���4��BaCl2��Һ��������ɫ���ݣ�����BaCl2��Һ��ʼ������4min����Һ����ǣ�������������ӣ�����������Ȼ�����Һ��Ѹ�����ɰ�ɫ������ʵ��2��֪��ȡ0.3g ����Na2SO3���壬�����м���10mL 2molL-1 HNO3���ٵ���4��BaCl2��Һ��������ɫ���ݣ�����BaCl2��Һ��ʼ������2h����Һ����ǣ�����������Ȼ�����Һ��+4����Ԫ�ػ��ϼ۱����������ʼ�����������������ӻ�Ѹ�����ɳ������ʴ�Ϊ��bd��
CuSO4+SO2��+2H2O����Ϊ̽��SO2��D���������ķ�Ӧ����һ��ʵ�鷢�֣����ְ�ɫ�����Ĺ����У�D��Һ��NO3-Ũ�ȼ������䣮�ݴ˵ó����ۣ�D�г��ְ�ɫ��������Ҫԭ����������Һ�ж�������ᱻ���������������ᣬ��ϱ����ӣ�Ҳ���������ᱵ�������ʴ�Ϊ�����������£���+4����Ԫ������(SO2��H2SO3)��O2��������SO42-��(2)��ȡ0.3g ����Na2SO3���壬�����м���10mL 2molL-1 ���ᣬ�ٵ���4��BaCl2��Һ��������ɫ����Ϊ�����������壬����BaCl2��Һ��ʼ������4min����Һ����ǣ�˵�����������������������������ᣬ��ϱ������������ᱵ��ɫ��������Ӧ�����ӷ���ʽΪ��2H++SO32-�TSO2+H2O��2SO2+O2+2Ba2++2H2O�T2BaSO4��+4H+��2H2SO3+O2+2Ba2+�T2BaSO4��+4H+���ʴ�Ϊ��2H++SO32-�TSO2+H2O��2SO2+O2+2Ba2++2H2O�T2BaSO4��+4H+��2H2SO3+O2+2Ba2+�T2BaSO4��+4H+������ʵ��1˵������������Ҳ��������+4����Ԫ�صĻ����ʵ��2˵��������Һ����������Ӷ�������Ӧ�������ã����ֳ���ʱ�䳤��ʵ��3��Ũ����������+4����Ԫ�ػ�����������������ӣ����ֳ����죬�Աȿ�֪������Ũ���ᶼ���������������ʴ�Ϊ����+4����Ԫ�����ʿɱ�O2��ŨHNO3��������̽��Cl-��NO3-�����Ӱ�죬i��ʵ��2��4�Աȣ��һ�����ۣ�Cl-�Ĵ��ڿ��Լӿ���Һ��+4����Ԫ�ص�������ʵ��4����Ҫ�ṩ��ʵ��1����ͬ��������ʵ��̽��������Ҫ0.01L��2mol/L=0.02mol���Ȼ��Ƶ�����=0.02mol��58.5g/mol=1.17g���Ա�ʵ��1�жϳ��ֳ�����ʱ�������ȡ0.3g ����Na2SO3��1.17gNaCl���壬�����м���10mL 2molL-1 HNO3���ٵ���4��BaCl2��Һ���۲���ֳ�����ʱ�䣬�ʴ�Ϊ��0.3g����Na2SO3��1.17gNaCl��ii��ʵ��1��4�Աȣ���ͬ������������ᣬ��������ͬ�����ֳ�����ʱ����������Һ�п죬�һ����������������Ӽ���+4����Ļ������������ʵ��1��4�Աȣ��һ�������ǣ�NO3-�Ĵ��ڿ��Լ�����Һ��+4����Ԫ�ص��������ʴ�Ϊ��NO3-�Ĵ��ڿ��Լ�����Һ��+4����Ԫ�ص��������ܶԱ�����ʵ��ȷ��ij��Һ�к���SO42-��ʵ�鷽���ǣ�ʵ��1��֪��ȡ0.3g ����Na2SO3���壬�����м���10mL 2molL-1 ���ᣬ�ٵ���4��BaCl2��Һ��������ɫ���ݣ�����BaCl2��Һ��ʼ������4min����Һ����ǣ�������������ӣ�����������Ȼ�����Һ��Ѹ�����ɰ�ɫ������ʵ��2��֪��ȡ0.3g ����Na2SO3���壬�����м���10mL 2molL-1 HNO3���ٵ���4��BaCl2��Һ��������ɫ���ݣ�����BaCl2��Һ��ʼ������2h����Һ����ǣ�����������Ȼ�����Һ��+4����Ԫ�ػ��ϼ۱����������ʼ�����������������ӻ�Ѹ�����ɳ������ʴ�Ϊ��bd��