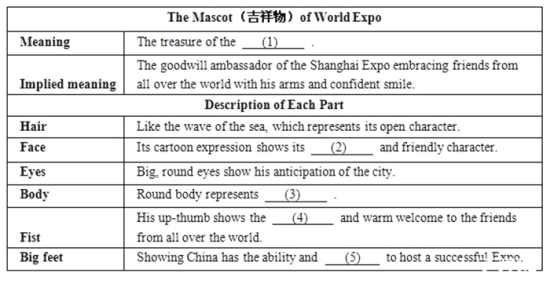
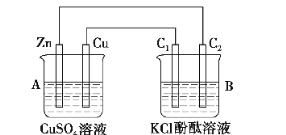
��Ŀ����
����Ŀ��(��)��![]() ��
��![]() ��H+��Cu2+��Ba2+��Ag+��Cl��������ѡ���ʵ���������ɵ���ʣ����ö��Ե缫������Һ���е�⡣
��H+��Cu2+��Ba2+��Ag+��Cl��������ѡ���ʵ���������ɵ���ʣ����ö��Ե缫������Һ���е�⡣
��1�������ֱ�ų�H2��O2ʱ������ʵĻ�ѧʽ������_______________________________��
��2�����������������������ų�O2������ʵĻ�ѧʽ������________________________________��
��3�������ֱ�ų����壬�������Ϊ1��1������ʵĻ�ѧʽ������_______________________________��
(��)��֪��Ӧ��Cu+H2SO4(ϡ)===CuSO4+H2��ͨ�����ܷ�������ش�
��4����˵���˷�Ӧ��һ������²��ܷ�Ӧ��ԭ��_______________________________________��
��5������ϡH2SO4��Һ��ͨ���ȵĿ���������ͭ�����ܽ⡣��д���йصĻ�ѧ����ʽ��_______________________________________________________��
��6����������ѧ����֪ʶ���跨ʹ�˷�Ӧ�ܷ���������ķ�����_______________________________����Ӧԭ����______________________________________________________��
���𰸡�(��)��1��H2SO4��HNO3��Ba(NO3)2
��2��AgNO3��Cu(NO3)2��CuSO4 ��3��HCl��BaCl2
(��)��4����ͭ�ڽ������˳�����������ĺ��棬���Բ�����ϡ���ᷴӦ�û������е���
��5��2Cu+O2+2H2SO4(ϡ) ![]() 2CuSO4+2H2O
2CuSO4+2H2O
��6����ͭ��������ʯī��������ϡH2SO4�����Һ��ɵ��� ������Ӧʽ��Cu2e===Cu2+��������Ӧʽ��2H++2e===H2��
��������(��)��1�����ˮ�ͣ�������ΪOH�ŵ磬����ΪH+�ŵ硣
��2������ΪOH�ŵ磬����Ϊ����H��Ľ��������ӷŵ硣
��3�����������Ϊ1��1�����ǵ��ˮ�͡�
(��)Cu���ڽ������˳���H�ĺ��棬��Cu��ϡH2SO4����Ӧ����Ƴɵ��أ�����Cu���������������ɷ�����ⷴӦ��Cu+H2SO4(ϡ)![]() CuSO4+H2����
CuSO4+H2����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�