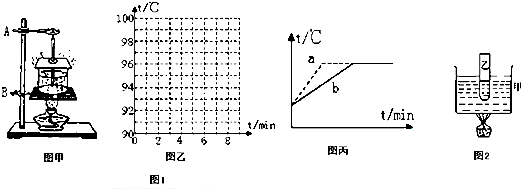
��Ŀ����
����3����ͬѧ��ʵ��������ˮ�ķ���ʵ�顱��Ϊ�˽�Լ��Դ�����̼���ʱ�䣬��ʦ�ṩ���dz��´�Լ90�����ҵ���ˮ��
��1���ڰ�װ������ʵ������ʱ����ѧ������˳���ǣ���ͼ�У����ȵ����̶�
��2���ž�ͬѧ��¼��ʵ������й۲쵽������ˮ����ʱ��ˮ��С��������;����
��3������ͬѧ�Ѽ��ȹ����еõ������ݼ�¼���±��У������ݿ�֪��ˮ�ķе�
��
��4������ͬѧ��ͬһ���ƾ��Ƽ���ˮ���Ⱥ��������Ρ��о�ˮ�ķ��ڡ�ʵ�飬�ֱ���ͼ����ʾ��a��b����ͼ�ߣ�ʵ�����ݼ���ͼ������ȷ�ģ��Է���һ�£�Ϊʲô����ͼ���в��죬��ԭ����

��1���ڰ�װ������ʵ������ʱ����ѧ������˳���ǣ���ͼ�У����ȵ����̶�
B
B
��λ�ã��ٵ����̶�A
A
��λ�ã���ѡ�A����B��������2���ž�ͬѧ��¼��ʵ������й۲쵽������ˮ����ʱ��ˮ��С��������;����
���
���
����������С�����䡱������ʱ�ƾ��Ƽ������ȣ�ˮ���¶����ֲ���
���ֲ���
��ѡ��������������ֲ��䡱������3������ͬѧ�Ѽ��ȹ����еõ������ݼ�¼���±��У������ݿ�֪��ˮ�ķе�
��
98
98
�森���ݼ�¼�����ݣ���ͼ���л���ˮ����ǰ���¶���ʱ��仯��ͼ��| ����ʱ��/min | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | �� |
| ˮ���¶�/�� | 90 | 92 | 94 | 96 | 98 | 98 | 98 | 98 | 98 | �� |
ˮ��������ͬ
ˮ��������ͬ
����������1��Ҫ������⣬��Ҫ���վƾ��ƺ��¶ȼƵ�ʹ�ù���֪��Ҫ�þƾ��Ƶ�������ȣ��¶ȼƵIJ�����Ҫ��ȫ��û��Һ���У����������������ں������ף�
��2��Һ�����ǰ�����������¶Ȳ������ߣ���������ʱ���ϱ�С��Һ�����ʱ�����������¶ȱ��ֲ��䣬��������ʱ��������
��3��Һ�����ʱ������¶Ⱦ�������Һ��ķе㣮
����������㣬����ƽ������������������
��4��Ҫ������⣬��ӷе�ı仯�ͼ���ʱ��ı仯�����з�����
��2��Һ�����ǰ�����������¶Ȳ������ߣ���������ʱ���ϱ�С��Һ�����ʱ�����������¶ȱ��ֲ��䣬��������ʱ��������
��3��Һ�����ʱ������¶Ⱦ�������Һ��ķе㣮
����������㣬����ƽ������������������
��4��Ҫ������⣬��ӷе�ı仯�ͼ���ʱ��ı仯�����з�����
����⣺��1����ʵ���У���Ҫ�þƾ��Ƶ�������ȣ�������Ҫ���ݾƾ��ƣ�����ȷ��B��λ�ã�Ȼ������¶ȼƵ�ʹ�ù���ȷ��A��λ�ã�
�ʴ�Ϊ��B��A��
��2��Һ�����ʱ�����������¶ȱ��ֲ��䣬��������ʱ��������
�ʴ�Ϊ������ֲ��䣮
��3����ͼ��֪��ˮ�µ���98��ʱ���������ߣ����������¶Ⱦ�������Һ��ķе㣬��ˮ�ķе���98�棬
�ʴ�Ϊ��98��
����������㣬����ƽ������������������
�ʴ�Ϊ��
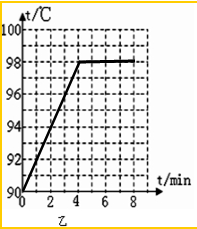
��4����ͼ��֪�����β����ķе���ͬ��ֻ��b���е�ļ���ʱ��ϳ���������Ҫԭ������Ϊ����ˮ��������ͬ��ɵģ�
�ʴ�Ϊ��ˮ��������ͬ��
�ʴ�Ϊ��B��A��
��2��Һ�����ʱ�����������¶ȱ��ֲ��䣬��������ʱ��������
�ʴ�Ϊ������ֲ��䣮
��3����ͼ��֪��ˮ�µ���98��ʱ���������ߣ����������¶Ⱦ�������Һ��ķе㣬��ˮ�ķе���98�棬
�ʴ�Ϊ��98��
����������㣬����ƽ������������������
�ʴ�Ϊ��

��4����ͼ��֪�����β����ķе���ͬ��ֻ��b���е�ļ���ʱ��ϳ���������Ҫԭ������Ϊ����ˮ��������ͬ��ɵģ�
�ʴ�Ϊ��ˮ��������ͬ��
������������Ҫ�ǹ۲�ˮ�ķ���ʵ�飬��Ҫ�����˴�ʵ���ʵ��Ŀ�ģ�֪��ʵ����Ҫ�۲�ˮ����ʱ��������ص㣮ͬʱ������ˮ����ʱ���ص㼰�¶ȼƵĶ������⣬֪��ˮ����ʱ�¶ȱ��ֲ��䣮���ܹ������ص������صķ���ͼ��

��ϰ��ϵ�д�
�����Ŀ