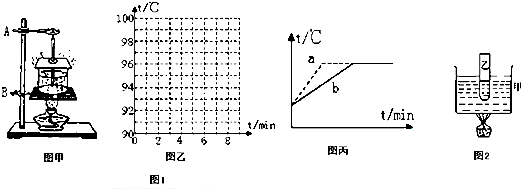
��Ŀ����
����3����ͬѧ��ʵ��������ˮ�ķ���ʵ�顱��Ϊ�˽�Լ��Դ�����̼���ʱ�䣬��ʦ�ṩ���dz��´�Լ90�����ҵ���ˮ��
��1���ž���¼ʵ���й۲쵽������ˮδ����ǰˮ��С��������;����
��2������ͬѧ�Ѽ��ȹ����еõ������ݼ�¼���±��У�����ݱ����е�������ͼ��ʾ���¶ȡ�����ʱ�������У��Ѽ��ȹ�����ˮ���¶ȱ仯ͼ��������

��3������ͬѧ��ͬһ���ƾ��Ƽ���ˮ���Ⱥ����˶��Ρ��о�ˮ�ķ��ڡ�ʵ�飬�ֱ���ͼ��ʾ��a��b����ͼ�ߣ�ʵ�����ݼ���ͼ������ȷ�ģ��Է���һ�£�Ϊʲô����ͼ���в��죬��ԭ����
��4����2�֣����ֺ��ܷ���λͬѧ�õ���ʵ����������ȣ�����ԭ���в����ܵ��ǣ�
A������ʹ�õ��¶ȼƲ�����B������ʹ�õľƾ��ƻ����д�С
C�����˲��Ե�ˮ�ʲ�һ����D�����˲���ʱ�¶ȼ�Һ����������
��1���ž���¼ʵ���й۲쵽������ˮδ����ǰˮ��С��������;����
��С
��С
������������С�����䡱����ͬ����ˮ����ʱ��ˮ��С��������;����
���
���
����ˮ�������ѣ��������ˮ����ɢ�������У���ʱ�ƾ��Ƽ������ȣ���ˮ���¶�
����
����
�����ƿ��ƾ��ƣ������¶ȼ���ʾ�¶���δ�½�����
����
����
����ֹͣ��������˵����Һ����ڵ�������Һ�����
�ﵽ�е�
�ﵽ�е�
�һ�Ҫ
��������
��������
����2������ͬѧ�Ѽ��ȹ����еõ������ݼ�¼���±��У�����ݱ����е�������ͼ��ʾ���¶ȡ�����ʱ�������У��Ѽ��ȹ�����ˮ���¶ȱ仯ͼ��������
| ����ʱ��/min | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | �� |
| ˮ���¶�/�� | 90 | 92 | 94 | 96 | 98 | 100 | 102 | 102 | 102 | 102 | �� |

��3������ͬѧ��ͬһ���ƾ��Ƽ���ˮ���Ⱥ����˶��Ρ��о�ˮ�ķ��ڡ�ʵ�飬�ֱ���ͼ��ʾ��a��b����ͼ�ߣ�ʵ�����ݼ���ͼ������ȷ�ģ��Է���һ�£�Ϊʲô����ͼ���в��죬��ԭ����
ˮ��������ͬ
ˮ��������ͬ
����4����2�֣����ֺ��ܷ���λͬѧ�õ���ʵ����������ȣ�����ԭ���в����ܵ��ǣ�
B
B
��A������ʹ�õ��¶ȼƲ�����B������ʹ�õľƾ��ƻ����д�С
C�����˲��Ե�ˮ�ʲ�һ����D�����˲���ʱ�¶ȼ�Һ����������
��������1��Ҫ�����������Ҫ���շ���ʱ�ͷ���ǰ��������ʱ���������������������������ǰ���������������������С��
Ҫ����ˮ����ʱ���ص㣺���ȵ��¶ȱ��ֲ��䣮
��2����㣬����ƽ������������������
��3����ͼ�����ҳ����𣬲���Ҫ֪��ˮ��������ʱ����ˮ�Ķ����йأ�
��4��Ҫ֪������ˮ�ķе�ߵ����¶ȼơ��������ˡ�ˮ���������ʶ��й�ϵ��
Ҫ����ˮ����ʱ���ص㣺���ȵ��¶ȱ��ֲ��䣮
��2����㣬����ƽ������������������
��3����ͼ�����ҳ����𣬲���Ҫ֪��ˮ��������ʱ����ˮ�Ķ����йأ�
��4��Ҫ֪������ˮ�ķе�ߵ����¶ȼơ��������ˡ�ˮ���������ʶ��й�ϵ��
����⣺��1��ˮ����ǰ���������������������С������ʱ�д��������ݲ���������������������ˮ��ѹǿ��С���������������
�ڷ��ڹ�������Ȼ�¶Ȳ��䣬��Ҫ�������ȣ�
ˮ���ڵ������ǣ��ﵽ�е㣬��Ҫ�������ȣ�
�ʴ�Ϊ����С������䣻���ڣ��ﵽ�е㣻�������ȣ�
��2������������㣬����ƽ�������������������ʴ�Ϊ��

��3����ͼ�����ݿ��Կ�����ˮ�ķе���ͬ��ֻ��b����ʱ�����õ�ʱ��ϳ�������Ϊb��ˮ����������a��ˮ��������
�ʴ�Ϊ��ˮ��������ͬ��
��4���ƾ��ƻ���Ĵ�Сֻ��Ӱ����������ʱ�䣬������Ӱ������ˮ�ķе㣮
��ѡB��
�ڷ��ڹ�������Ȼ�¶Ȳ��䣬��Ҫ�������ȣ�
ˮ���ڵ������ǣ��ﵽ�е㣬��Ҫ�������ȣ�
�ʴ�Ϊ����С������䣻���ڣ��ﵽ�е㣻�������ȣ�
��2������������㣬����ƽ�������������������ʴ�Ϊ��

��3����ͼ�����ݿ��Կ�����ˮ�ķе���ͬ��ֻ��b����ʱ�����õ�ʱ��ϳ�������Ϊb��ˮ����������a��ˮ��������
�ʴ�Ϊ��ˮ��������ͬ��
��4���ƾ��ƻ���Ĵ�Сֻ��Ӱ����������ʱ�䣬������Ӱ������ˮ�ķе㣮
��ѡB��
����������ʱ̽��ˮ�ķ��ڣ���Ҫ������ˮ����ǰ�ͷ���ʱ�������ڵ�������ͬʱ��������ˮ�ķ���ͼ��Ļ��������й�����ķ�����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ