��Ŀ����
�ڡ��۲�ˮ�ķ��ڡ�ʵ���У�
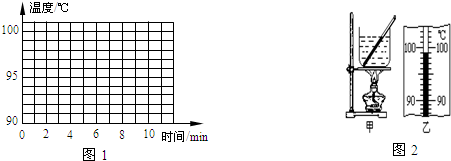
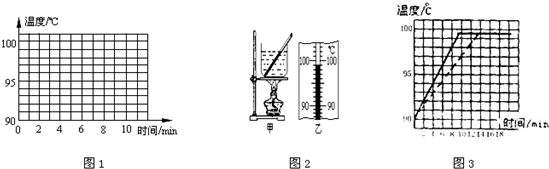
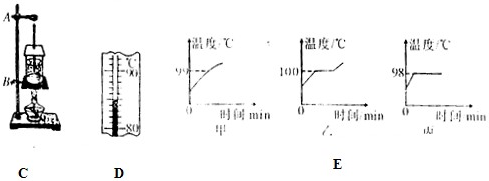
��1����ͼ��ʾ����С��ͬѧ���¶ȼƲ�С�ձ���ˮ�ij���ʱ�IJ���ͼ��a�Dz������̣�b�Ƕ������̣�c�Ƕ�ȡ���¶ȣ�
��aͼ�в����Ĵ�����
��bͼ�ж����Ĵ�����
����������ȷ������cͼ��֪��ʱ�ձ���ˮ��ʵ���¶���

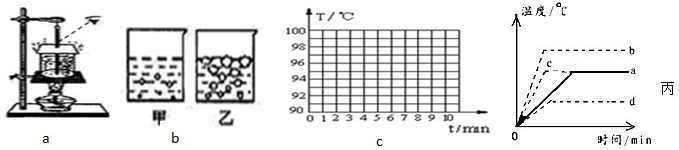
��2���ڡ�̽��ˮ���ڡ���ʵ���У��۲쵽ͼ��d������e����ʾ�������龰������Ϊ
��3��ʵ�����㻹�ܹ۲쵽��Щʵ����������д����������
��
��4��С��ͬѧ��������ͼ��ʾ��װ����ʵ��ʱ�����ִӿ�ʼ��ˮ���ȵ�ˮ��ʼ�������õ�ʱ�����������������ҳ����ܴ��ڵ�ԭ��д�����ּ��ɣ�
��
��1����ͼ��ʾ����С��ͬѧ���¶ȼƲ�С�ձ���ˮ�ij���ʱ�IJ���ͼ��a�Dz������̣�b�Ƕ������̣�c�Ƕ�ȡ���¶ȣ�
��aͼ�в����Ĵ�����
�¶ȼƵIJ����ݽӴ��˱���
�¶ȼƵIJ����ݽӴ��˱���
����bͼ�ж����Ĵ�����
�¶ȼƵIJ�����û���뱻��Һ���ֽӴ�
�¶ȼƵIJ�����û���뱻��Һ���ֽӴ�
������������ȷ������cͼ��֪��ʱ�ձ���ˮ��ʵ���¶���
48
48
�森
��2���ڡ�̽��ˮ���ڡ���ʵ���У��۲쵽ͼ��d������e����ʾ�������龰������Ϊ
d
d
ͼ��ˮ����ʱ���龰����3��ʵ�����㻹�ܹ۲쵽��Щʵ����������д����������
��
����ʱˮ���ڲ��ͱ���ͬʱ�������ҵ���������
����ʱˮ���ڲ��ͱ���ͬʱ�������ҵ���������
��������ǰ�¶ȼƵ�ʾ���������ߣ����ں�������ȶ��¶ȼƵ�ʾ�����ֲ���
����ǰ�¶ȼƵ�ʾ���������ߣ����ں�������ȶ��¶ȼƵ�ʾ�����ֲ���
����4��С��ͬѧ��������ͼ��ʾ��װ����ʵ��ʱ�����ִӿ�ʼ��ˮ���ȵ�ˮ��ʼ�������õ�ʱ�����������������ҳ����ܴ��ڵ�ԭ��д�����ּ��ɣ�
��
ˮ�ij��½ϵ�
ˮ�ij��½ϵ�
����ˮ�϶�
ˮ�϶�
���������ڡ��۲�ˮ�ķ��ڡ�ʵ���У�������ȷ�ķ���ʹ���¶ȼƣ�����ȷ������ʵ��ɹ��Ĺؼ�֮һ���۲�ͼ�¼ʵ������Ҳ�DZ�ʵ��Ӧ��ɵ���ҪĿ�ģ�Ϊ�����̸�ˮ���ȵ�ʱ�䣬�������ˮ�ij��ºͼ�С�����Ͻ��иĽ���
����⣺��1���ٶ�aͼ��֪������������¶ȼƵIJ����ݽӴ��˱��ף�
�ڶ�bͼ��֪������������¶ȼƵIJ�����û����Һ���ֽӴ���
��cͼ���¶ȼƵ�ʾ��Ϊ48�棻
��2����Ϊˮ����ʱ����ˮ���¶Ⱦ��ﵽ�е㣬ˮ������������֮���Խ��ѹǿԽС�����ݻ�Խ����ðԽ����ˣ�ͼd��ˮ����ʱ���龰��
��3��ʵ���л��ܹ۲쵽�������У��ٷ���ʱˮ���ڲ��ͱ���ͬʱ�������ҵ��������ڷ���ǰ�¶ȼƵ�ʾ���������ߣ����ں�������ȶ��¶ȼƵ�ʾ�����ֲ��䣮
��4��ˮ���ȵ�ʱ��ϳ������ܴ��ڵ�ԭ���У���ˮ�ij��½ϵͣ���ˮ�϶ࣨ�����ϴ�
�ʴ�Ϊ����1�����¶ȼƵIJ����ݽӴ��˱��ף����¶ȼƵIJ�����û���뱻��Һ���ֽӴ�����48��
��2��d��
��3���ٷ���ʱˮ���ڲ��ͱ���ͬʱ�������ҵ��������ڷ���ǰ�¶ȼƵ�ʾ���������ߣ����ں�������ȶ��¶ȼƵ�ʾ�����ֲ��䣻
��4����ˮ�ij��½ϵͣ���ˮ�϶ࣨ�����ϴ�
�ڶ�bͼ��֪������������¶ȼƵIJ�����û����Һ���ֽӴ���
��cͼ���¶ȼƵ�ʾ��Ϊ48�棻
��2����Ϊˮ����ʱ����ˮ���¶Ⱦ��ﵽ�е㣬ˮ������������֮���Խ��ѹǿԽС�����ݻ�Խ����ðԽ����ˣ�ͼd��ˮ����ʱ���龰��
��3��ʵ���л��ܹ۲쵽�������У��ٷ���ʱˮ���ڲ��ͱ���ͬʱ�������ҵ��������ڷ���ǰ�¶ȼƵ�ʾ���������ߣ����ں�������ȶ��¶ȼƵ�ʾ�����ֲ��䣮
��4��ˮ���ȵ�ʱ��ϳ������ܴ��ڵ�ԭ���У���ˮ�ij��½ϵͣ���ˮ�϶ࣨ�����ϴ�
�ʴ�Ϊ����1�����¶ȼƵIJ����ݽӴ��˱��ף����¶ȼƵIJ�����û���뱻��Һ���ֽӴ�����48��
��2��d��
��3���ٷ���ʱˮ���ڲ��ͱ���ͬʱ�������ҵ��������ڷ���ǰ�¶ȼƵ�ʾ���������ߣ����ں�������ȶ��¶ȼƵ�ʾ�����ֲ��䣻
��4����ˮ�ij��½ϵͣ���ˮ�϶ࣨ�����ϴ�
�������ڡ��۲�ˮ�ķ��ڡ�ʵ���У�Ҫ���������¶ȼƵ�ʹ�ù�����ȷʵ������Ĺ۲��ص㣬�������ʵ���д��ڵ����⣬��ʵ����б�Ҫ�ĸĽ���ֻ���������ǵ�ʵ����������ȫ����ߣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��ͼa��С����С���ڹ۲조ˮ�ķ��ڡ�ʵ���е��龰����ˮ�����˽ϳ�ʱ���ˮ�ŷ��ڣ����Ǽ�¼���������±���ʾ��
��ͼa��С����С���ڹ۲조ˮ�ķ��ڡ�ʵ���е��龰����ˮ�����˽ϳ�ʱ���ˮ�ŷ��ڣ����Ǽ�¼���������±���ʾ��
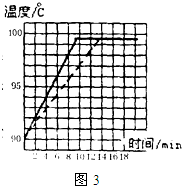 �ڡ��۲�ˮ�ķ��ڡ�ʵ���У���ˮ������90��ʱ��С��ͬѧ��ʼ��ʱ��ÿ��1min��¼һ��ˮ���¶ȣ��йؼ�¼�������£�
�ڡ��۲�ˮ�ķ��ڡ�ʵ���У���ˮ������90��ʱ��С��ͬѧ��ʼ��ʱ��ÿ��1min��¼һ��ˮ���¶ȣ��йؼ�¼�������£�