��Ŀ����
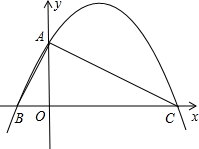 ��2012•��������֪����ͼ����ƽ��ֱ������ϵ�У�Rt��ABC��б��BC��x���ϣ�ֱ�Ƕ���A��y����������ϣ�A��0��2����B��-1��0����
��2012•��������֪����ͼ����ƽ��ֱ������ϵ�У�Rt��ABC��б��BC��x���ϣ�ֱ�Ƕ���A��y����������ϣ�A��0��2����B��-1��0������1�����C�����ꣻ
��2�����A��B��C����������ߵĽ���ʽ�ͶԳ��
��3�����P��m��n�����������ڵ�һ�������ϵĵ㣬��PAC�����ΪS����S����m�ĺ�����ϵʽ������ʹS���ʱ��P�����ꣻ
��4���������߶Գ����ϣ��Ƿ���������ĵ�M��ʹ�á�MPC��PΪ������3������ʹS���ʱ�ĵ㣩Ϊ���������Σ������ڣ���ֱ��д����M�����ꣻ�������ڣ���˵�����ɣ�
��������1��Rt��ABC�У�AO��BC����֪����OA��OB�ij�������Ӱ���������OC�ij���Ҳ�͵õ��˵�C�����꣮
��2�����ô���ϵ��������ȷ�������ߵĽ���ʽ����x=-
����������ߵĶԳ��ᣮ
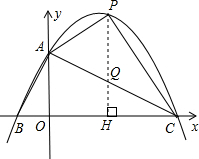
��3���������ֱ��AC�Ľ���ʽ������P��x��Ĵ��ߣ���ֱ��AC��Q����֪�������ߺ�ֱ��AC����ʽ������£���m��ʾ����P��Q�����꣬�����������ľ���ֵ��Ϊ�߶�PQ�ij�����S=
AC•PQ���ݴ���ù���S��m�ĺ�����ϵʽ�����ݺ��������ʼ���ȷ��S���ʱ��P�����꣮
��4�����������M�����꣬Ȼ���г���MPC�����߳��������������ǵ��������Σ����ݢ�MP=MC����MP=PC����MC=PC�г���ʽ��⼴�ɣ�
��2�����ô���ϵ��������ȷ�������ߵĽ���ʽ����x=-
| b |
| 2a |
��3���������ֱ��AC�Ľ���ʽ������P��x��Ĵ��ߣ���ֱ��AC��Q����֪�������ߺ�ֱ��AC����ʽ������£���m��ʾ����P��Q�����꣬�����������ľ���ֵ��Ϊ�߶�PQ�ij�����S=
| 1 |
| 2 |
��4�����������M�����꣬Ȼ���г���MPC�����߳��������������ǵ��������Σ����ݢ�MP=MC����MP=PC����MC=PC�г���ʽ��⼴�ɣ�
����⣺��1����Rt��ABC�У�AO��BC��OA=2��OB=1��
��OC=
=4��
��C��4��0����
��2���������ߵĽ���ʽ��y=a��x+1����x-4���������A�����꣬�ã�
a��0+1����0-4��=2��a=-
�������ߵĽ���ʽ��y=-
��x+1����x-4��=-
x2+
x+2���Գ��� x=
��
��3����ֱ��AC�Ľ���ʽΪ��y=kx+2�������C��4��0�����ã�
4k+2=0��k=-
��ֱ��AC��y=-
x+2��
����P��PQ��x����H����ֱ��AC��Q����P��m��-
m2+
m+2����
��S����AOHP=
[2+��-
m2+
m+2��]m=-
m3+
m2+2m��
S��PHC=
��4-m����-
m2+
m+2��=
m3-
m2+2m+4��
S��AOC=
��4��2=4��
S=S����AOHP+S��PHC-S��AOC=-m2+4m=-��m-2��2+4��
�൱m=2���� P��2��3��ʱ��S��ֵ���
��4�������⣬��M��
��b������֪P��2��3����C��4��0�������У�
MP2=b2-6b+
��MC2=b2+
��PC2=13��
��MP=MCʱ��b2-6b+
=b2+
����� b=
��
��MP=PCʱ��b2-6b+
=13����� b=
��
��MC=PCʱ��b2+
=13����� b=��
��
���ϣ����ڷ���������M�㣬������Ϊ ��
��
������
��
������
��
������
��
������
��-
����
��OC=
| OA2 |
| OB |
��C��4��0����
��2���������ߵĽ���ʽ��y=a��x+1����x-4���������A�����꣬�ã�
a��0+1����0-4��=2��a=-
| 1 |
| 2 |
�������ߵĽ���ʽ��y=-
| 1 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
��3����ֱ��AC�Ľ���ʽΪ��y=kx+2�������C��4��0�����ã�
4k+2=0��k=-
| 1 |
| 2 |

��ֱ��AC��y=-
| 1 |
| 2 |
����P��PQ��x����H����ֱ��AC��Q����P��m��-
| 1 |
| 2 |
| 3 |
| 2 |
��S����AOHP=
| 1 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 4 |
| 3 |
| 4 |
S��PHC=
| 1 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 4 |
| 7 |
| 4 |
S��AOC=
| 1 |
| 2 |
S=S����AOHP+S��PHC-S��AOC=-m2+4m=-��m-2��2+4��
�൱m=2���� P��2��3��ʱ��S��ֵ���
��4�������⣬��M��
| 3 |
| 2 |
MP2=b2-6b+
| 37 |
| 4 |
| 25 |
| 4 |
��MP=MCʱ��b2-6b+
| 37 |
| 4 |
| 25 |
| 4 |
| 1 |
| 2 |
��MP=PCʱ��b2-6b+
| 37 |
| 4 |
6��
| ||
| 2 |
��MC=PCʱ��b2+
| 25 |
| 4 |
3
| ||
| 2 |
���ϣ����ڷ���������M�㣬������Ϊ ��
| 3 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
6+
| ||
| 2 |
| 3 |
| 2 |
6-
| ||
| 2 |
| 3 |
| 2 |
3
| ||
| 2 |
| 3 |
| 2 |
3
| ||
| 2 |
��������Ŀ��Ҫ���������ô���ϵ����ȷ����������ʽ����������������Լ����������ε��ж������ʣ����ƣ�4���⣺�ڵ��������ε����͵ײ�ȷ��������£�һ��Ҫ���з������ۣ�

��ϰ��ϵ�д�
�����Ŀ
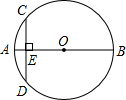 ��2012•��������ͼ��ABΪ��O��ֱ����CDΪ��O��һ���ң�CD��AB������ΪE����֪CD=6��AE=1�����0�İ뾶Ϊ
��2012•��������ͼ��ABΪ��O��ֱ����CDΪ��O��һ���ң�CD��AB������ΪE����֪CD=6��AE=1�����0�İ뾶Ϊ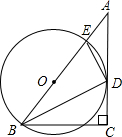 ��2012•������ģ����֪����ͼ����Rt��ABC�У���C=90�㣬��ABC��ƽ����BD��AC�ڵ�D��DE��DB��AB�ڵ�E��
��2012•������ģ����֪����ͼ����Rt��ABC�У���C=90�㣬��ABC��ƽ����BD��AC�ڵ�D��DE��DB��AB�ڵ�E��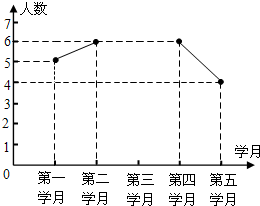 ��2012•������ģ��Ϊ������ý�ѧ�ĸ�ٽ�ѧ��ȫ�淢չ��ijУ�������пθ�ʵ�飮ѧУΪ�˹������б���ͻ����ͬѧ��ÿѧ�½��С�У֮�ǡ���ѡ�����2012���Ա��꼶��ѧ�����ѧ�µĻ�����������ͳ�ƣ����Ƴ������²�����������ͳ��ͼ��
��2012•������ģ��Ϊ������ý�ѧ�ĸ�ٽ�ѧ��ȫ�淢չ��ijУ�������пθ�ʵ�飮ѧУΪ�˹������б���ͻ����ͬѧ��ÿѧ�½��С�У֮�ǡ���ѡ�����2012���Ա��꼶��ѧ�����ѧ�µĻ�����������ͳ�ƣ����Ƴ������²�����������ͳ��ͼ��