��Ŀ����
����Ŀ����Һ�����ǵ���������������ء�
��1�������������ʷֱ����ˮ�У���ֽ��裬�ܵõ���Һ����_____������ţ���
A�Ҵ� B���� C��� D����
��2����ʢ��ˮ���ձ��м�������ij�����ʣ��γ���Һ�����У��¶��½�������������_____������ţ���
A���� B����� C�Ȼ��� D��������
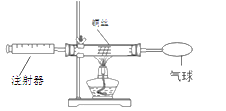
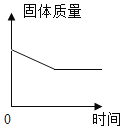
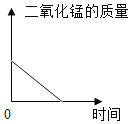
��3������غ��Ȼ�淋��ܽ��������ͼһ��ʾ������M����2�������е�һ�֡�ij��ѧ��ȤС���ͬѧ��������ͼ����ʾʵ�顣

��t1��ʱ���Ȼ�淋��ܽ��Ϊ_____g��
���ձ�l����Һ�����ʵ���������Ϊ_____��
�۹���M��_____��
�����й���ͼ�����ձ������ʵ�˵����ȷ����_____������ţ���
a.������Һ��������160g
b.�����������У�ֻ�������ϲ���Һ�DZ�����Һ
c.��ʹ���еĹ����ܽ⣬�ɲ��ü�ˮ�����µķ���
d.�����ϲ���Һ��������������һ����������Һ����������������
��4��ȡһ������������������ͭ�Ļ�������100g��������Ϊ9.8%��ϡ���ᣬǡ����ȫ��Ӧ��������Һ���ܼ���������_____g��
���𰸡�A B 40 30% ![]() ac 92
ac 92
��������
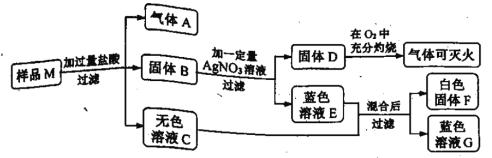
��1��A���Ҵ�������ˮ���ʿɵõ���Һ���ʷ������⣻
B������ΪҺ���Ҳ�����ˮ�����ͻ��ˮ�γ���Һ���ʲ��������⣻
C�����Ϊ�����Ҳ�����ˮ����ۻ��ˮ�γ���Һ���ʲ��������⣻
D������Ϊ�����Ҳ�����ˮ�����ۻ��ˮ�γ���Һ���ʲ��������⣻
���A
��2��A����������ˮ�Ȳ�����Ҳ�����ȣ���Һ�¶Ȳ��䣻�ʲ��������⣻
B�����������ˮ���ȣ���ʹ��Һ�¶��½����ʷ������⣻
C���Ȼ�������ˮ�Ȳ�����Ҳ�����ȣ���Һ�¶Ȳ��䣻�ʲ��������⣻
D���ռ�����ˮ���ȣ���Һ�¶����ߣ��ʲ��������⣻
���B
��3������ͼ1��֪���¶�Ϊt1��ʱ���Ȼ�淋��ܽ��Ϊ40g�����40g
���ձ�l����Һ���������֪Mȫ���ܽ⣬��Һ��M�����ʵ�����Ϊ60g����Һ����Ϊ140g+60g=200g���ձ�l����Һ��������������Ϊ��![]() �����30%
�����30%
��MΪ����ػ��Ȼ���е�һ�֣�����������t2�沢����40gˮ���ձ��в����������t2��ʱM���ܽ�Ȳ�С��60g���Ȼ�鱗�������������M������أ�KNO3�������KNO3
��a. t2��ʱ��������Һǡ����M����أ�KNO3���ı�����Һ��������160g��˵����ȷ���ʷ������⣻
b.��Ϊ��������Һ����Ϊǡ���DZ�����Һ����Ϊ������Һ��˵�����ʲ��������⣻
c.��ʹ���еĹ����ܽ⣬���ü�ˮ�����µķ��������У�˵����ȷ���ʷ������⣻
d.����ϲ���Һ����������������һ���Ȣ����Һ������������������Ϊֻ��˵�˽��£�û���ᵽ���µ����١棬�ʲ���ȷ�����ܽ�ȣ���Ϊ������Һ���������������п��ܱȢ����Һ������������С���ʲ��������⣻
��4����:100g��������Ϊ9.8%��ϡ������ˮ������Ϊ:100g-100![]() 9.8%=90.2g��
9.8%=90.2g��
100g��������Ϊ9.8%��ϡ���������������Ϊ: 100![]() 9.8%=9.8g��
9.8%=9.8g��
9.8g�����е���Ԫ������Ϊ:![]()
�����е�0.2g��Ԫ����ȫת������ˮ����ˮ�Ļ�ѧʽH2O����֪����2H![]() H2O����2
H2O����2![]() 18��
18��
��0.2g��Ԫ��ת����ˮ������Ϊx��
����![]() ��x=1.8g
��x=1.8g
���Է�Ӧ��������Һ���ܼ���������:90.2g+1.8g=92g
��������Һ���ܼ���������92g�����92

 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�����Ŀ���±��Ǽ��ֿ�ȼ���ڳ�ѹʱ�ķе㣻
��ȼ�� | CH4 | C2H6 | C3H8 | X | C5H12 |
�е�/�� | 164 | 88.6 | 42.1 | 0.5 | 36.1 |
��1����C3H8�У�̼Ԫ������Ԫ�ص�������Ϊ_____________����������ȣ���
��2����C3H8�У�̼Ԫ�ص���������Ϊ_____________����������ȷ��0.1%����
��3��45gC2H6��_____________gC5H12����̼Ԫ��������ȡ�
��4�������ϱ��п�ȼ����ӽṹ�ϵĹ��ɣ��Ʋ�X�Ļ�ѧʽΪ_____________��
����Ŀ���ᡢ����������������о��й㷺����;��
��1����ѧʵ������ʧȥ��ǩ��ϡ���ᡢ�������ơ���̪��̼���ơ����ᱵ����ƿ��ɫ��Һ���ֽ��������ţ�![]() ��Ȼ��������Ͻ���ʵ�飬�䲿���������±���
��Ȼ��������Ͻ���ʵ�飬�䲿���������±���
ʵ�� |
|
|
|
|
���� | �������� | �������� | �������� | ��Һ��� |
��д����Һ![]() �����ʵĻ�ѧʽ��
�����ʵĻ�ѧʽ��![]() _____��
_____��![]() _____��
_____��
��д����Һ![]() ��
��![]() ��Ӧ�Ļ�ѧ����ʽ_____��
��Ӧ�Ļ�ѧ����ʽ_____��
��2�����Ȼ��ƺ��Ȼ��ƵĻ����![]() ����ˮ���ټ���
����ˮ���ټ���![]() ������������Ϊ
������������Ϊ![]() ��̼������Һ��ǡ����ȫ��Ӧ����û��������Ԫ�ص���������Ϊ_____�������ȷ��
��̼������Һ��ǡ����ȫ��Ӧ����û��������Ԫ�ص���������Ϊ_____�������ȷ��![]() ����
����