题目内容
【题目】学完二氧化碳的性质之后,实验兴趣小组的同学对紫色石蕊试纸变红色的实验进行了深入的研究。
(提出问题)是什么物质使紫色石蕊试纸变红色的?
(提出假设)(1)是水使紫色石蕊试纸变红色;
(2)是二氧化碳使紫色石蕊试纸变红色
(3)是碳酸使紫色石蕊试纸变红色。
(设计实验)
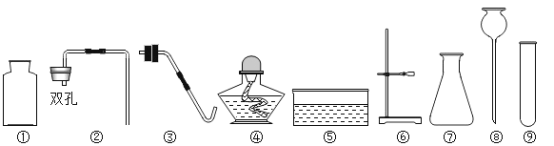
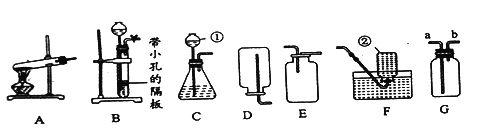
他们设计了如图所示的实验。请你根据他们的实验,回答下列问题:(提示浓硫酸具有吸水性)
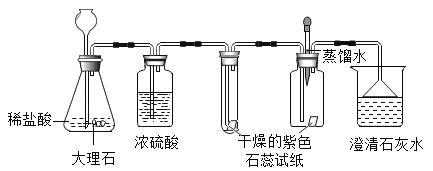
(1)写出用稀盐酸和大理石制取CO2的化学方程式______。
(2)①实验过程中,试管内紫色石蕊试纸的颜色始终没有变化。
②集气瓶中胶头滴管中的蒸馏水,在二氧化碳气体生成前滴至紫色石蕊试纸上,未见试纸发生颜色变化,当有二氧化碳通过时发现湿润的紫色石蕊试纸变红,此现象说明:______。
③在烧杯中出现的现象是什么?______
(得出练论)假设(3)成立。
【答案】CaCO3+2HCl=CaCl2+H2O+CO2↑ 二氧化碳与水反应生成碳酸,碳酸使紫色石蕊试纸变红色 澄清的石灰水变浑浊
【解析】
(1)大理石的主要成分是碳酸钙,碳酸钙与盐酸反应生成氯化钙、水和二氧化碳;化学方程式:CaCO3+2HCl=CaCl2+H2O+CO2↑;
(2)②实验过程中,试管内紫色石蕊试纸的颜色始终没有变化,说明二氧化碳不能使紫色石蕊试纸变红色;集气瓶中胶头滴管中的蒸馏水,在二氧化碳气体生成前滴至紫色石蕊试纸上,未见试纸发生颜色变化,说明二氧化碳不能使紫色石蕊试纸变红色,当有二氧化碳通过时发现湿润的紫色石蕊试纸变红,此现象说明二氧化碳与水反应生成碳酸,碳酸使紫色石蕊试纸变红色;
③二氧化碳与氢氧化钙反应生成碳酸钙白色沉淀和水,所以烧杯中观察到澄清的石灰水变浑浊。

练习册系列答案
 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案
相关题目