Ő‚ńŅńŕ»›
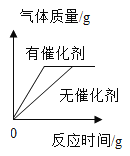
°ĺŐ‚ńŅ°Ņ Ķ—ť “”–ľ◊°Ę““ŃĹ∆Ņĺ√÷√Ķń«‚—űĽĮń∆ĻŐŐŚ£¨ń≥—ßŌį–°◊ťő™Ńň—–ĺŅ∆šĪš÷ «ťŅŲ£¨ĹÝ––Ńň»ÁŌ¬ Ķ—ť£ļ£®ĶÁ◊”≥” ĺ żĶ•őĽő™g£©
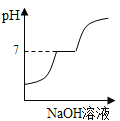
£®1£©Ķő»Ž◊Ō…ę Į»Ô»‹“ļļů»‹“ļő™ļž…ę£¨ňĶ√ų∑ī”¶ļů»‹“ļ≥ _____–‘°£
£®2£©…Ō Ų Ķ—ť≤ķ…ķĶń∂Ģ—űĽĮŐľ∆ÝŐŚ÷ ŃŅő™_____g°£
£®3£©ľ∆ň„ľ◊∆ŅĻŐŐŚ—ý∆∑÷–ŐľňŠń∆Ķń÷ ŃŅ∑÷ ż_____°£
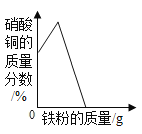
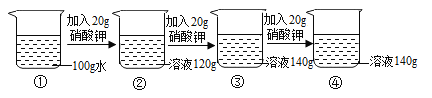
£®4£©ń≥Õ¨—ßŃŪ»°10 g““∆Ņ÷–ĶńĻŐŐŚ—ý∆∑£¨”√100 g 15%ĶńŌ°ŃÚňŠįīÕ¨—ý∑Ĺ∑®ĹÝ–– Ķ—ť£¨ňŻ»Ōő™≤ĽĻ‹ĻŐŐŚ—ý∆∑Īš÷ ≥Ő∂»»Áļő£¨Ō°ŃÚňŠľ”»Žļů£¨∂ľ≤Ľ–Ť“™ Ļ”√ Į»Ô»‹“ļ£¨«Žľ∆ň„ňĶ√ųňŻ◊Ų≥ŲīňŇ–∂ŌĶń‘≠“Ú£ļłýĺ›Na2CO3£ęH2SO4=Na2SO4£ęH2O£ęCO2°Ł£¨√Ņ106∑›÷ ŃŅĶńŐľňŠń∆ÕÍ»ę∑ī”¶–Ť98∑›ĶńH2SO4£¨100 g15%ĶńŌ°ŃÚňŠ÷–H2SO4Ķń÷ ŃŅő™_____g£¨ňý“‘_____°£
°ĺīūįł°ŅňŠ 2.2 53% 15 ľ”»Ž100g15%ĶńŌ°ŃÚňŠĹÝ–– Ķ—ť£¨Ō°ŃÚňŠ“Ľ∂®ĻżŃŅ£¨Ļ Ō°ŃÚňŠľ”»Žļů≤Ľ–Ť“™ľ” Į»Ô»‹“ļ
°ĺĹ‚őŲ°Ņ
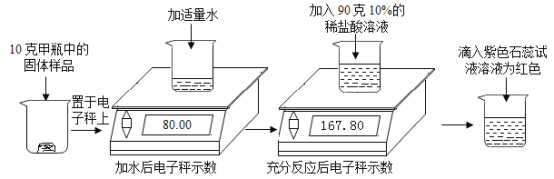
£®1£©Ķő»Ž◊Ō…ę Į»Ô»‹“ļļů»‹“ļő™ļž…ę£¨ňĶ√ų∑ī”¶ļů»‹“ļ≥ ňŠ–‘°£
£®2£©łýĺ›÷ ŃŅ ōļ„∂®¬…£¨≤ķ…ķĶń∂Ģ—űĽĮŐľ∆ÝŐŚ÷ ŃŅő™80.00g+90g-167.80g=2.2g°£
£®3£©…ŤĻŐŐŚ—ý∆∑÷–ŐľňŠń∆Ķń÷ ŃŅő™x£¨‘Ú
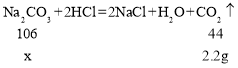
![]()
x=5.3g
ĻŐŐŚ—ý∆∑÷–ŐľňŠń∆Ķń÷ ŃŅ∑÷ żő™![]() °£
°£
£®4£©100 g15%ĶńŌ°ŃÚňŠ÷–ŃÚňŠĶń÷ ŃŅő™![]() £¨ľ”»Ž100g15%ĶńŌ°ŃÚňŠĹÝ–– Ķ—ť£¨ŌŻļńŐľňŠń∆Ķń÷ ŃŅīů”ŕ10g,Ō°ŃÚňŠ“Ľ∂®ĻżŃŅ£¨Ļ Ō°ŃÚňŠľ”»Žļů≤Ľ–Ť“™ľ” Į»Ô»‹“ļ°£
£¨ľ”»Ž100g15%ĶńŌ°ŃÚňŠĹÝ–– Ķ—ť£¨ŌŻļńŐľňŠń∆Ķń÷ ŃŅīů”ŕ10g,Ō°ŃÚňŠ“Ľ∂®ĻżŃŅ£¨Ļ Ō°ŃÚňŠľ”»Žļů≤Ľ–Ť“™ľ” Į»Ô»‹“ļ°£

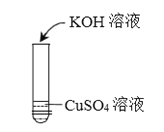
°ĺŐ‚ńŅ°Ņń≥–ň»§–°◊ťÕ¨—ß‘ŕ◊ŲĹū Ű”Ž—ő»‹“ļ∑ī”¶Ķń Ķ—ť Ī,∑ĘŌ÷ń∆∑Ň»ňŃÚňŠÕ≠»‹“ļļů,≤Ę√Ľ”–ļž…ęĻŐŐŚőŲ≥Ų,∂Ý «»‹“ļ÷–”–őř…ę∆ÝŇ›ļÕņ∂…ę≥ŃĶŪ…ķ≥…°£Õ¨—ß√«∂‘≤ķ…ķ’‚--“ž≥£Ō÷ŌůĶń‘≠“ÚĹÝ––ŐĹĺŅ°£
Õ¨—ß√«Ĺę≥š∑÷∑ī”¶ļůĶńĽžļŌőÔĻż¬ň,Ķ√ĶĹőř…ę¬ň“ļA°£
[≤ť‘ń◊ ŃŌ]ń∆”Žňģ∑ī”¶…ķ≥…«‚—űĽĮń∆°£–ī≥Ų«‚—űĽĮń∆”ŽŃÚňŠÕ≠∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ_________________________°£
[ĹÝ–– Ķ—ť]Õ¨—ß√«∂‘≤ķ…ķĶń∆ÝŐŚļÕőř…ę¬ň“ļAĶń≥…∑÷ĹÝ––»ÁŌ¬ŐĹĺŅ°£
Ķ—ťŐĹĺŅ | ľ◊◊ť | ““◊ť |
[ŐŠ≥Ųő Ő‚] | ≤ķ…ķĶń∆ÝŐŚ « ≤√ī? | őř…ę¬ň“ļAĶń»‹÷ ≥…∑÷ « ≤√ī? |
[◊ų≥Ų≤¬ŌŽ] | ≤¬ŌŽ“Ľ:H2 ≤¬ŌŽ∂Ģ:O2 ≤¬ŌŽ»ż:CO | ≤¬ŌŽĘŔ:Na2SO4 ≤¬ŌŽĘŕ:Na2SO4°ĘNaOH ≤¬ŌŽĘŘ:Na2SO4°ĘCuSO4 |
[ Ķ—ť—ť÷§] | –°ņŲ»Ōő™≤ĽŅ…ń‹…ķ≥…CO,ņŪ”… «_____________________________°£–°”Ę ’ľĮ“Ľ ‘Ļ‹≤ķ…ķĶń∆ÝŐŚ£¨Ļ‹ŅŕŌÚŌ¬“∆ĹŁĺ∆ĺęĶ∆Ľū—ś£¨ŐżĶĹľ‚»ŮĶńĪ¨√ý…ý | »°…ŔŃŅőř…ę¬ň“ļA”ŕ ‘Ļ‹÷–,ŌÚ∆š÷–Ķőľ”ľłĶőőř…ę∑”Ő™»‹“ļ,ĻŘ≤žĶĹ________________________°£ |
[ Ķ—ťĹŠ¬Ř] | ≤¬ŌŽ______________________≥…ŃĘ | ≤¬ŌŽĘŕ≥…ŃĘ |
[∑īňľĹĽŃų]–°√ų»Ōő™őř–Ť Ķ—ť÷§√ų,¬ň“ļA÷–“Ľ∂®√Ľ”–CuSO4,‘≠“Ú «________________________°£
[Õō’Ļ”¶”√]”√ŃÚňŠÕ≠ļÕ ĮĽ“ňģŇš÷∆Ň©“©≤®∂Ż∂ŗ“ļ Ī£¨≤Ľń‹”√________________________(ŐÓ◊÷ńł)»›∆ų Ę∑Ň°£
A Õ≠÷∆»›∆ų
B ī…÷∆»›∆ų
C Őķ÷∆»›∆ų
D ≤£Ńß»›∆ų
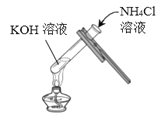
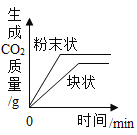
°ĺŐ‚ńŅ°Ņń≥–ň»§–°◊ť‘ŕ Ķ—ť “÷–ŐĹĺŅ«‚—űĽĮľōĶń–‘÷ £¨«Žń„≤ő”ŽňŻ√«ĶńŐĹĺŅ°£
£® Ķ—ťĻż≥Ő”Ž∑÷őŲ£©
Ķ—ť“Ľ | Ķ—ť∂Ģ | Ķ—ť»ż | |
Ķ—ť≤Ŕ◊ų |
|
|
|
Ķ—ťŌ÷Ōů | _______ | ______ | őř√ųŌ‘Ō÷Ōů |
Ķ—ťĹŠ¬Ř | «‚—űĽĮľōń‹”ŽŃÚňŠÕ≠∑Ę …ķ∑ī”¶ | «‚—űĽĮľōń‹”Ž¬»ĽĮÔß∑Ę …ķ∑ī”¶ | «‚—űĽĮľō≤Ľń‹”ŽŃÚňŠ ∑Ę…ķ∑ī”¶ |
£® Ķ—ť∑īňľ”ŽÕō’Ļ£©
ĘŔń≥Õ¨—ß»Ōő™ Ķ—ť»żĶńĹŠ¬Ř≤Ľ’ż»∑°£ňŻłńĹÝŃňł√ Ķ—ť∑Ĺįł£¨ĹŤ÷ķ”ŕňŠľÓ÷ł ĺľŃ£¨Õ®Ļż√ųŌ‘ĶńŌ÷Ōů÷§√ų«‚—űĽĮľōń‹”ŽŃÚňŠ∑Ę…ķ∑ī”¶°£ľÚ ŲňŻĶń Ķ—ť∑Ĺįł£ļ_____£®–ī≥Ų≤Ŕ◊ų∑Ĺ∑®ļÕŌ÷Ōů£©°£
Ęŕ∂‘”ŕőř√ųŌ‘Ō÷ŌůĶńĽĮ—ß∑ī”¶£¨Ņ…Õ®Ļżľž—ť”––¬őÔ÷ …ķ≥…ĽÚľž—ť_____Ķń∑Ĺ∑®ņī÷§√ųőÔ÷ ľš∑Ę…ķŃňĽĮ—ß∑ī”¶°£
°ĺŐ‚ńŅ°ŅŌ¬ĪŪ «¬»ĽĮľōļÕŌűňŠľō‘ŕ≤ĽÕ¨ő¬∂»Ō¬Ķń»‹Ĺ‚∂»°£łýĺ›ĪŪ÷– żĺ›,«ŽĽōīūŌ¬Ń–ő Ő‚:
ő¬∂»/°„C | 0 | 20 | 40 | 60 | 80 | 100 | |
»‹Ĺ‚∂»/g | KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 | 246 |
KCl | 27.6 | 34.0 | 40.0 | 45.5 | 51.1 | 56.7 | |
(1)‘ŕňý ĺĶń◊ÝĪÍ÷Ĺ…ŌĽś÷∆≥Ų¬»ĽĮľōĶń»‹Ĺ‚∂»«ķŌŖ______________°£
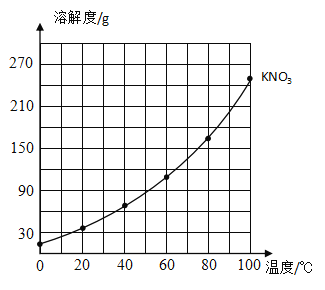
(2)Ī»ĹŌŃĹ÷÷őÔ÷ Ķń»‹Ĺ‚∂»«ķŌŖ£¨»‹Ĺ‚∂» ‹ő¬∂»”įŌžĪšĽĮĹŌ–°Ķń «______________£¨ő¬∂»īů‘ľ‘ŕ___________°„C Ī,ŃĹ÷÷őÔ÷ Ķń»‹Ĺ‚∂»īů–°ŌŗĶ»°£
(3)60°„C Ī,105gĶńKNO3Ī•ļÕ»‹“ļ÷–ļ¨”–Ķń»‹÷ Ķń÷ ŃŅő™____________°£
(4)40°„C Ī,ŌÚ…’Ī≠÷–ľ”»Ž50gňģļÕ25gKClĻŐŐŚ,≥š∑÷»‹Ĺ‚ļůĶ√ĶĹĶń»‹“ļ «_____________(ŐÓ°įĪ•ļÕ°ĪĽÚ°į≤ĽĪ•ļÕ°Ī)»‹“ļ,∆š»‹÷ ÷ ŃŅ∑÷ żő™_____________(ĺę»∑ĶĹ0.1% )°£
(5)»ŰKNO3÷–Ľž”–…ŔŃŅĶńKCl ,ŐŠīŅKNO3Ķń∑Ĺ∑® «_____________°£