��Ŀ����
����ͼʾ�龰�ͷ�������д�����⣬�����������ݵ���ա�
���龰l�� Ҫ����һ������������һ���������Ȼ�����Һ��Ӧ����ô����?
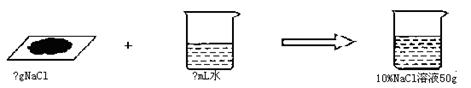
(1)���ⷶ�� ����10����NaCl��Һ50g����NaCl��������ˮ������ֱ��Ƕ���?
(2)���Ƹ���Һ�IJ�������
�ټ��㣺
�ڳ�������������ƽ��ȡ������壬����______mL��ѡ�10������50������100��������Ͳ��ȡ�����ˮ��
���ܽ⣺���Ƶõ��Ȼ��ƹ�������ձ��У��ٵ�����ȡ��ˮ���ò�������ֽ��裻
��װƿ������õ���Һ�����Լ�ƿ�������ϱ�ǩ��
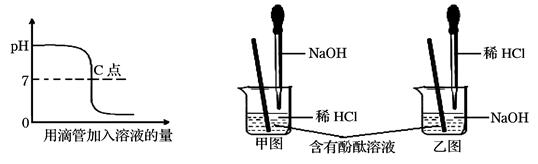
���龰2�� ��֪һ������������һ������������������Һ��һ�����������ᷴӦ���õ�ֻ��һ�����ʵ���Һ�����㷴Ӧ����Һ����������������
(1)��д��Ŀ______________________________________________________________��
(2)�������
���龰l�� Ҫ����һ������������һ���������Ȼ�����Һ��Ӧ����ô����?

(1)���ⷶ�� ����10����NaCl��Һ50g����NaCl��������ˮ������ֱ��Ƕ���?
(2)���Ƹ���Һ�IJ�������
�ټ��㣺
�ڳ�������������ƽ��ȡ������壬����______mL��ѡ�10������50������100��������Ͳ��ȡ�����ˮ��
���ܽ⣺���Ƶõ��Ȼ��ƹ�������ձ��У��ٵ�����ȡ��ˮ���ò�������ֽ��裻
��װƿ������õ���Һ�����Լ�ƿ�������ϱ�ǩ��
���龰2�� ��֪һ������������һ������������������Һ��һ�����������ᷴӦ���õ�ֻ��һ�����ʵ���Һ�����㷴Ӧ����Һ����������������

(1)��д��Ŀ______________________________________________________________��
(2)�������
���龰1��50��2�֣�
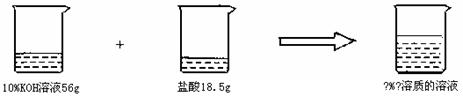
���龰2����1����д��Ŀ����֪56g10��������������Һ��18.5gijŨ�ȵ�����ǡ����ȫ��Ӧ����������Һ���ʵ����������� ��1�֣�
��2��������� ��3�֣�
�⣺m(KOH)=56g��10��=5.6g
�跴Ӧ���ɵ�KCl������Ϊx
KOH + HCl =" KCl" + H2O
56 74.5
5.6g x
�б���ʽ���x=7.45g
��(KCl)=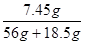 ��100��=10��
��100��=10��
���ԡ�
���龰2����1����д��Ŀ����֪56g10��������������Һ��18.5gijŨ�ȵ�����ǡ����ȫ��Ӧ����������Һ���ʵ����������� ��1�֣�
��2��������� ��3�֣�
�⣺m(KOH)=56g��10��=5.6g
�跴Ӧ���ɵ�KCl������Ϊx
KOH + HCl =" KCl" + H2O
56 74.5
5.6g x
�б���ʽ���x=7.45g
��(KCl)=
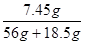 ��100��=10��
��100��=10�����ԡ�
Ҫѡ����������ȡҺ�������ӽ����ݻ�����Ͳ��ͬʱҪ������ȡ�����Ҫ��
��֪�������Ƶ����������ݻ�ѧ����ʽ�б���ʽ���н��
��֪�������Ƶ����������ݻ�ѧ����ʽ�б���ʽ���н��

��ϰ��ϵ�д�
�����Ŀ

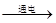 Cu2O+H2�������ô˷�������72kg��������ͭ����������ˮ�������Ƕ��٣�
Cu2O+H2�������ô˷�������72kg��������ͭ����������ˮ�������Ƕ��٣�