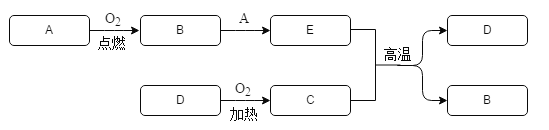
��Ŀ����
����Ŀ���������Ʒ�����ຬ�����������������������°�����Ϣ��Ϊý���ע�Ļ��⣬��֪�����������Ļ�ѧʽΪC12H7Cl3O2����������������������˵����ȷ���ǣ� ��
A. ��������̼���⡢�ȡ�����Ԫ��������Ϊ12�U7�U3�U2
B. ��֪�ڸ������У�����-1�ۣ��⡢���ļ�̬��������ɵ�һ�ֳ�����������Ԫ�ؼ�̬��ͬ����̼�Ļ��ϼ�Ϊ+4��
C. ��������һ���л���������12��̼ԭ�ӡ�7����ԭ�ӡ�3����ԭ�Ӻ�һ�������ӹ���
D. ��������̼Ԫ�ص���������ԼΪ49.74%
���𰸡�D
��������
A���ɻ�ѧʽΪC12H7Cl3O2��̼���⡢�ȡ���Ԫ�ص�������Ϊ��12��12������1��7������35.5��3������16��2��=144��7��106.5��32��ѡ��˵�����ʲ��������⣻
B����֪�ڸ������У�����-1�ۣ��⡢���ļ�̬��������ɵ�һ�ֳ�����������Ԫ�ؼ�̬��ͬ������Ԫ����-1�ۣ���Ԫ����+1�ۣ���̼Ԫ�صĻ��ϼ�Ϊx����12x+��+1����7+��-1����3+��-1����2=0����x=0�ۣ�ѡ��˵�����ʲ��������⣻
C�������������Ǻ�̼Ԫ�صĻ���������л����1��������12��̼ԭ�ӡ�7����ԭ�ӡ�3����ԭ�Ӻ�2����ԭ�ӹ��ɵģ����������ӣ�ѡ��˵�����ʲ��������⣻
D����������̼Ԫ�ص���������ԼΪ![]() ��ѡ��˵����ȷ���ʷ������⣻
��ѡ��˵����ȷ���ʷ������⣻
��ѡD

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д� �Ͻ�ƽ��У����ϵ�д�
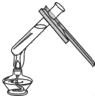
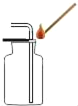
�Ͻ�ƽ��У����ϵ�д�����Ŀ��ijУ��ѧ��ȤС��ι��Ƽ���������Ϣ���������������о���
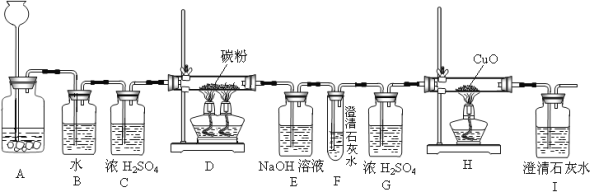
����Ʒ��ԭ�����ó������������Ƽ������������Ʒ�������Na2CO3���ͻ���NH4Cl��
����ԭ���ǣ���NH3��CO2ͨ�뱥��ʳ��ˮ�еõ�NaHCO3��NH4Cl�Ļ�����ӦΪ��NaCl�����ͣ�+ NH3 + CO2 + H2O = NaHCO3��+ NH4Cl�������NaHCO3���壬�����Ƶô��
���������̣�

��������ϣ�
��1�� NH4Cl ![]() NH3��+HCl��
NH3��+HCl��
��2�� ��֪20��ʱ�й����ʵ��ܽ�����£�����ָ1���ˮ�����ܽ�����������
���� | NaCl | NaHCO3 | NH4Cl | NH3 | CO2 |
�ܽ�� | 36.0g | 9.6g | 37.2g | 710 | 0.9 |
���������ۣ�:
��1�����ҵ��ؼ���һ���ǣ��ڼ�ѹ�������²����͵İ���ˮ��ͨ�������̼���壬��Һ�л���̼�����ƾ����������Է�����������Ҫ��ѹ������ԭ���ǣ�________�������м��������������Ŀ����________������II�����ƽ�________��
��2����Ӧ���з�����������Ӧ��д�����е�һ����ѧ����ʽ��________________��
��3����Ӧ���еõ�����Ļ�ѧ����ʽΪ��_________________________________��
��4���������������п�ѭ��ʹ�õ���________������ţ���
A ����C B ��ҺD C ������þ D ����NH4Cl
��5���ڰ��ҵ����ʣ����Ȼ����Һʱ��Ϊ�β�ֱ�������ᾧ�����Ȼ�粒���?_________��
�����ȷ����
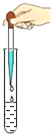
��1����ȡһ�������Ĵ�����Ʒ������γ�ּ��Ⱥ��ٳ��أ������ޱ仯��
��2����ȡ����������Ʒ��������ˮ����Ʒ��ȫ�ܽ⣬�����Һ�м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ������������ʵ���ȷ��������Ʒ��������________��д��ѧʽ����
��3��ij�о���ѧϰС��Ը��������Ĵ����Ʒ���м�⡣ȡ22�˸���Ʒ���ձ��У���ˮ�����ܽ⣬Ȼ����μ���������������Ϊ14.6%��ϡ���Ტ������
���ȷ����ķ�Ӧ�ǣ�Na2CO3+HCl=NaHCO3+NaCl��Ȼ�����ķ�Ӧ�ǣ�NaHCO3+HCl= NaCl+H2O+CO2����
�������������ձ�����Һ���������ϡ���������Ĺ�ϵ��ͼ����ʾ��

�������ͼ���ṩ����Ϣ���������Ӧ�����Ķ�����̼������________���������Ʒ�д������������________(�������ðٷ�����ʾ,������С�����һλ)
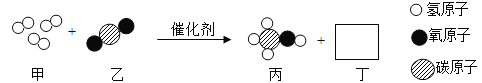
����Ŀ��һ�������£������������ܱ������ڳ�ַ�Ӧ����÷�Ӧǰ������ʵ��������£�
������ | �Ҵ� | ���� | ������̼ | ˮ | X |
��Ӧǰ����/g | 2.3 | 4 | 0 | 0 | 0 |
��Ӧ������/g | 0 | 0 | 2.2 | 2.7 | ���� |
A����Ӧ��X������Ϊ1.5g B��X��һ������̼Ԫ�غ���Ԫ��
C��X��һ������̼Ԫ�غ���Ԫ�� D��X������Ԫ�ص���������1��1