��Ŀ����
����Ŀ��ij��ѧ����С���ͬѧ����ʵ����ʵ��ʱ����һƿ��ǩ�������ɫ��Һ(��ͼ)����ƿ��Һ��ʲô���ʣ�����ʦ��ָ���£�ͬѧ������������벢���������µ�ʵ��̽��������ڿհ״������Ӧ���ݡ�
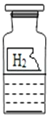
��������룩����1��ˮ ����2��_____��
�����ʵ�飩���Թ���ȡ������ɫ��ĩ_____�������е������ɫ��Һ�����������������ݡ�
������ˮ���ռ�һ�Թ����壻���ô����ǵ�ľ�������Թ��У�
�۳�����Ӧǰ���ɫ������������䣻
��ʵ�������ڢ��е�������_____��֤����Һ����_____��
��ʵ����ۣ���ɫ��ĩ�ڴ�ʵ��ķ�Ӧ����_____���ã�ԭ����_____(��1��2)������
���𰸡��������� �������� �����ǵ�ľ����ȼ �������� �� 2
��������
[�������]
����1��ˮ�Ļ�ѧʽ��H2O�����Һ�������ˮ ����2���������⻯ѧʽ��H2O2�����Һ������ǹ������⡣����������⡣
[���ʵ��]
���Թ���ȡ������ɫ��ĩ�������̣������е������ɫ��Һ�����������������ݡ�����������̡�
[ʵ������]�ڢ��е������Ǵ����ǵ�ľ����ȼ������Ϊ��������ֽ�������������֤����Һ���ǹ������⡣��������ǵ�ľ����ȼ���������⡣
[ʵ�����]
��ɫ��ĩ�ڴ�ʵ��ķ�Ӧ��������ã�ԭ����2�������������2��

����Ŀ������Ƭ���ϱ����£�
�ɷ� | ������ | ̼������ | ά����C | ���� | ɫ���㾫 |
ÿƬ������4 g/Ƭ�� | δ֪ | δ֪ | 0.2 g | δ֪ | ������ |
����Ƭ�����¿�ˮ�У�����ˮ���ϣ������������ݣ�������Һ����������ɿڡ���֪��������Ļ�ѧʽΪC6H8O7����Է�������Ϊ192������Ƭ����ˮʱ��̼�����Ƹպ�����������ȫ��Ӧ������Na3C6H5O7��
��1������Ƭ��ˮ�в�������Ļ�ѧʽΪ_____������Ƭ����������̼�����Ƶ�������Ϊ_____��
��2����ȡ5.00 g����Ƭ����200.00 g�¿�ˮ�У�������Ƭ��ȫ�ܽ⣬���ٲ�����������Һ����Ϊ203.68 g��ͨ������ȷ������Ƭ�����������������_____��
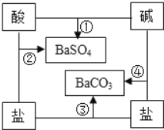
����Ŀ��ijʵ��С�����÷�����Һ�Ʊ�K2SO4

��1����CaCO3�гɷ�ĩ��Ŀ���� ��
��2�����������п�ѭ��ʹ�õ�������CO2�� ����д��ѧʽ����
��3����Ӧ����������ʵ��ܽ�����±�������Ϊ��Ӧ���ڳ�������ʵ�ֵ�ԭ����
���� | KCl | K2SO4 | NH4Cl | M |
�ܽ��/g��25���� | 34.0 | 11.1 | 37.2 | 19.5 |
��4������ˮ���ñ���K2SO4��Һϴ�ӷ�Ӧ�����þ����Ŀ���� ��Ϊ����˾����Ƿ�ϴ�Ӹɾ�����ȡ���һ��ϴ��Һ���ȼ��� ��ѡ����ţ���ͬ���������ã������ϲ���Һ�еμ� ���۲������жϣ�
a��AgNO3��Һ b��������BaCl2��Һ c��������Ba��NO3��2��Һ
����Ŀ��ij��ѧ��ȤС���ͬѧ��ͨ����ѯ��ʦ��������������Һ��Ũ���ᷴӦ���Ʊ�һ������SO2��Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O������ʦ�������ṩ��һƿ����������Һ����֪��ƿ��Һ����ʱ����ܽϳ�����֪�Ƿ���ʡ���ȤС���ͬѧ�ֳɼס�����С��Ը�ƿ����������Һ�ɷֽ���ʵ��̽����
��������⣩�ٸ�ƿ��Һ�����ʵijɷ���ʲô���ڸ�ƿ��Һ���������Ƶ����������Ƕ��٣�
���������ϣ�
��1��Na2SO3�н�ǿ��ԭ�ԣ��ڿ������ױ�����������2Na2SO3+O2=2Na2SO4����2��Na2SO3�����ᷴӦ����SO2���壻
��3��SO32-��SO42-������Ba2+��Ӧ������ɫ������BaSO3������ϡ���ᡣ
���������룩
����1û�б��ʣ��ɷ���Na2SO3������2��ȫ���ʣ��ɷ���Na2SO4��
����Ϊ�������еIJ���3______��
��ʵ��̽��I���ס�������ֱ����ʵ��̽����Һ�Ƿ���ʣ�
С�� | ʵ����� | ���� | ���� |
���� | ȡ������Ʒ���Թ��м������ϡ���� | �������� | û�б��ʣ�����Na2SO3 |
���� | ȡ������Ʒ���Թ��м����Ȼ�����Һ���ټ�������ϡ���� | ______ | �Ѳ��ֱ��� |
�����ۣ���ͬѧ���ɼ��鷽����������������______��
��ʵ��̽����
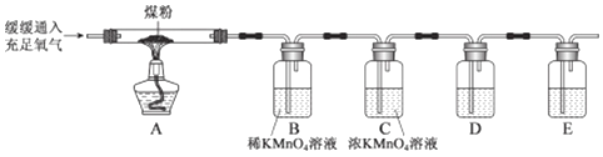
�����������ʵ��ⶨNa2SO3��Һ������������������ע�������ж�����̼��Ӱ����Բ��ƣ�
��1������װ�ò����װ�������ԣ�����ƿ�з���126g����Ʒ��
��2��ʵ��ǰ����Cװ�õ�������
��3���رջ���K����ע��������Ũ���������ٲ������ݣ�
��4������K����������һ�����ĵ������رջ���K��
��5���ٴγ���Cװ�õ��������ֱȷ�Ӧǰ����6.4g��
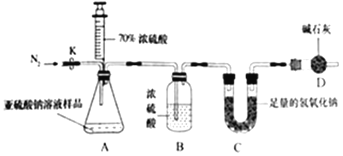
Dװ�õ�����Ϊ______�����������Һ��Na2SO3����������Ϊ______��
�����ͣ���û�н��е�4����������������������Һ����������������ʵ�ʵ�______������ƫС������ƫ��������
����˼���ɴ˿ɵó���������������ʱӦ______��