��Ŀ����
����Ŀ��ij��������������(��Ҫ��FeO��Fe2O3������һ������SiO2)����������ˮ��������������(FeSO47H2O)���乤���������£�
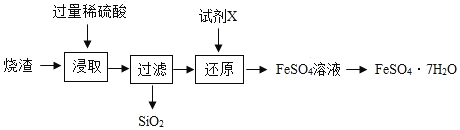
�������ϣ�SiO2������ˮ��Ҳ����ϡ���ᷴӦ��
(1)����ȡ�������У�FeO��Fe2O3��ϡ���ᷢ����Ӧ�Ļ�ѧ����ʽ�ֱ�ΪFeO+H2SO4=FeSO4+H2O��________��
(2)Ϊ�������ȡ������ķ�Ӧ�������ɲ�ȡ�ľ����ʩ��________(дһ����������������������ʹ�ô�����)��
(3)����ԭ�������Ŀ���ǽ�Fe3+ת��ΪFe2+���Լ�X����SO2��Fe�����Լ�X��Fe��ת��ԭ��ΪFe+Fe2(SO4)3=3FeSO4���������ԭ������Һ��pH������������ԭ����________��
(4)��FeSO4��Һ�õ�FeSO47H2O�Ĺ����а���������________�Ȳ�����������������������IJ��������оƾ��ơ�________��
���𰸡� Fe2O3+3H2SO4=Fe2(SO4)3+3H2O ���������Ũ�ȡ������¶ȡ������������ �����Ĺ��������ᣬ��Һ���Լ��� �ᾧ ������
��������(1)����ȡ�������У�FeO��Fe2O3��ϡ���ᷢ����Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ��FeO+H2SO4=FeSO4+H2O��Fe2O3+3H2SO4=Fe2(SO4)3+3H2O��(2)Ϊ��ߡ���ȡ������ķ�Ӧ���ʣ��ɲ�ȡ�ľ����ʩ�У����������Ũ�ȣ������¶ȣ��������������(3)���Լ�X��Fe��ת��ԭ��ΪFe+Fe2(SO4)3=3FeSO4����á���ԭ������Һ��pH����������ԭ���������Ĺ��������ᣬ��Һ���Լ�����(4)��FeSO4��Һ�õ�FeSO47H2OӦ�����������ᾧ�Ȳ�����������������������IJ��������оƾ��ơ���������

����Ŀ��ijʵ��С�����÷�����Һ�Ʊ�K2SO4���о�CaSO42H2O���ȷֽ�IJ��
��һ��K2SO4���Ʊ�
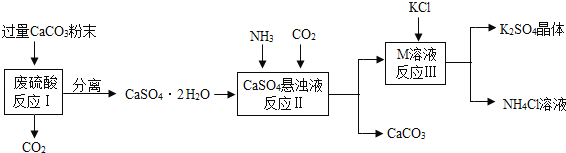
��1����CaCO3�гɷ�ĩ��Ŀ����___________________________________��
��2��M���ʵĻ�ѧʽΪ__________��
��3����Ӧ����������ʵ��ܽ�����±���
���� | KCl | K2SO4 | NH4Cl | M |
�ܽ��/g��25�棩 | 34.0 | 11.1 | 37.2 | 19.5 |
��Ӧ���ڳ�������ʵ�ֵ�ԭ����_______________________________________��
��4�����������п�ѭ��ʹ�õ�������CO2��_____________����д��ѧʽ����
�������о�CaSO42H2O���ȷֽ�IJ���
��5���������CaSO42H2O�г�����CaCO3�����������ȥCaCO3��
�÷�Ӧ�Ļ�ѧ����ʽ_______________________________________________��
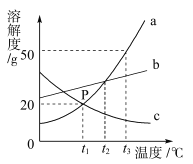
��6��Ϊ�˲ⶨCaSO42H2O��CaCO3��������x��y��ʵ��С��������ͼ��ʾ��װ�ã��г�����ʡ�ԣ�����ʵ�顣ע����ʯ�ҵ���Ҫ�ɷ�ΪNaOH��CaO��
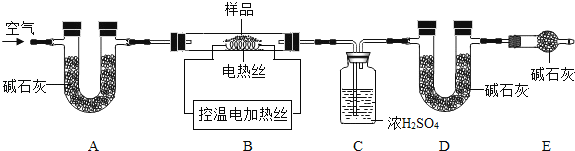
�� ʵ��ǰ����Ҫ________________����װ����Ʒ��װ��A��������_________________��
�� ��֪��CaSO42H2O��160������CaSO4��1350��ʱCaSO4��ʼ�ֽ⣻ CaCO3��900��ʱ�ֽ���ȫ��
�ֿ���Bװ���¶�900�����ʵ�鲢�ɼ����������ݣ�
a����Ӧǰ����������Ʒ������m1g b����Ӧ�������й��������Ϊm2g
c��װ��Cʵ�������m3g d��װ��Dʵ�������m4g
ijͬѧѡ��b��d��c��d����������x��y��ֵ������װ��E����ʵ��ⶨ�����______�����ƫ����ƫС������Ӱ�족������Ϊ����ѡ��������________________��ѡ����ţ������������Ҳ�����x��y��ֵ��
��7��CaSO42H2O���Ȼ���ʧȥ�ᾧˮ��ȡ����CaSO42H2O����3.44g�����ڣ�5����ʵ��װ��B�н��м��ȣ��ⶨ�����������¶ȵı仯�����ͼ��ʾ��
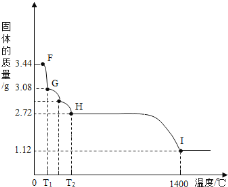
��G�����Ļ�ѧʽ��_________________��
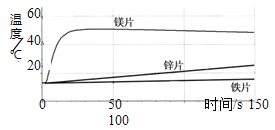
����Ŀ��ʵ�鼼��ѵ���У�С��ͬѧ������ͭ��ϡ�����ַ�Ӧ��ķ�Һ�м���һ����������������Һ����������������С��ͬѧ����ʦ��ָ���¶�����ͭ��ϡ�����ַ�Ӧ��ķ�Һ������ʵ�飺���ֱ�ȡ50g��Һ���������������Ũ�ȵ�����������Һ������ʵ���������ͼ�����£�

ʵ������ | ��һ�� | �ڶ��� | ������ |
��������������Һ����/g | 50 | 100 | 80 |
��������������/g | 0.98 | 2.94 | 2.94 |
�����������Ϣ�ش��������⣺
(1)����ͭ��ĩ��ϡ���ᷴӦ������Ϊ___________________��
(2)��Һ�е�������_______________(�ѧʽ) ���������Һ������ͭ��������������____________��(д���������)
(3)�����������У�ֻ��һ����������������Һ���Һǡ����ȫ��Ӧ������ͼ��a����ֵΪ____________��