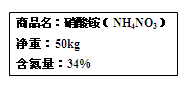
��Ŀ����
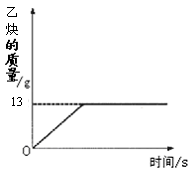
��10�֣���Ȳ��C2H2����һ����Ҫ����ԭ�ϣ�ʵ���ҳ���̼���ƣ�CaC2����ˮ��Ӧ��ȡ��ij��ѧ��ȤС��ȡһ��������̼������90gˮ��Ӧ����������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ����ش��������⣺
��1��̼���ƣ�CaC2����ˮ��Ӧ�Ļ�ѧ����ʽΪ��CaC2 + 2H2O �� + C2H2��������X��һ�ּ��X�Ļ�ѧʽΪ ��
+ C2H2��������X��һ�ּ��X�Ļ�ѧʽΪ ��
��2��̼������Ca��CԪ��������Ϊ ��
��3����Ӧ������������Ȳ������Ϊ_______g��
��4��ȡ��̼���Ƶ�����Ϊ���٣�

��1��̼���ƣ�CaC2����ˮ��Ӧ�Ļ�ѧ����ʽΪ��CaC2 + 2H2O ��
 + C2H2��������X��һ�ּ��X�Ļ�ѧʽΪ ��
+ C2H2��������X��һ�ּ��X�Ļ�ѧʽΪ ����2��̼������Ca��CԪ��������Ϊ ��
��3����Ӧ������������Ȳ������Ϊ_______g��
��4��ȡ��̼���Ƶ�����Ϊ���٣�
�� Ca(OH)2��1�֣� �� 40:24��2�֣� ��13��2�֣� ��4��32g
��1�����������غ㶨�ɵ�Ԫ���غ㣬���Ʋ�X�Ļ�ѧʽ���Ƹ���̼��ƵĻ�ѧʽΪCaCO3���������ԭ������������Ԫ�ص������ȣ�3����ͼ��֪��Ӧ������������Ȳ������Ϊ13 g��(4) ���ݷ���ʽ������Ȳ����������������Ҫ̼���Ƶ�������
(4)��̼���Ƶ�����Ϊx��������1�֣�
CaC2 + 2H2O ��Ca(OH)2 + C2H2��������1�֣�
64 26
x 13g������1�֣�
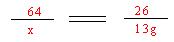 ������1�֣�
������1�֣�
��֮�ã�x=32g������1�֣�
�� ȡ��̼���Ƶ�����Ϊ32g������ʹ�ȫ�Ը�1�֣����д�����֡���
(4)��̼���Ƶ�����Ϊx��������1�֣�
CaC2 + 2H2O ��Ca(OH)2 + C2H2��������1�֣�
64 26
x 13g������1�֣�
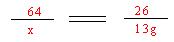 ������1�֣�
������1�֣���֮�ã�x=32g������1�֣�
�� ȡ��̼���Ƶ�����Ϊ32g������ʹ�ȫ�Ը�1�֣����д�����֡���

��ϰ��ϵ�д�
�����Ŀ
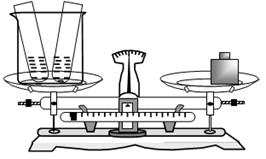
 С�ո��ݡ����Dz�������Ϊ4.3g������õ�����̼��Ƶ���������Ϊ ��
С�ո��ݡ����Dz�������Ϊ4.3g������õ�����̼��Ƶ���������Ϊ ��