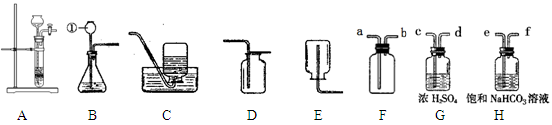
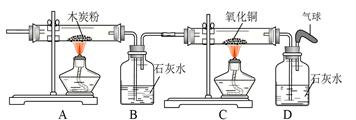
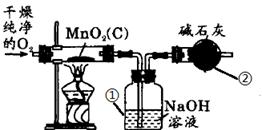
��Ŀ����
�����ǵ���Ҫ�ɷ���̼��ƣ�Ϊ�˲ⶨ������̼��Ƶ�����������С�պ�С��ͬѧ����������ʵ�顣��ش�������⣺��1������������ϴ�������ﲢ��������ձ��Ȼ�����ձ��м����������ᣬ�ڵ����Ϻܿ��������С���ݲ��������ռ���������ͨ�����ʯ��ˮ�У�ʯ��ˮ����ǡ��ݴ˿��жϣ��ռ����������к��� ��
��2��ʵ����̺Ͳⶨ�����ʵ������������ʾ��
 С�ո��ݡ����Dz�������Ϊ4.3g������õ�����̼��Ƶ���������Ϊ ��
С�ո��ݡ����Dz�������Ϊ4.3g������õ�����̼��Ƶ���������Ϊ ��
С�¸��ݡ���Ӧ�����м��ٵ���������Ҳ����˵�����̼��Ƶ�����������������������������������������д��С�µļ�����̺ͽ����
��3��С�պ�С�¸��ݲ�ͬ��������õ�����̼��Ƶ��������������Բ������������п��ܵ�ԭ��
��2��ʵ����̺Ͳⶨ�����ʵ������������ʾ��
 С�ո��ݡ����Dz�������Ϊ4.3g������õ�����̼��Ƶ���������Ϊ ��
С�ո��ݡ����Dz�������Ϊ4.3g������õ�����̼��Ƶ���������Ϊ ��С�¸��ݡ���Ӧ�����м��ٵ���������Ҳ����˵�����̼��Ƶ�����������������������������������������д��С�µļ�����̺ͽ����
��3��С�պ�С�¸��ݲ�ͬ��������õ�����̼��Ƶ��������������Բ������������п��ܵ�ԭ��
������������̼-------------------------------------------------------1��
������57% ------------------------------------------------------------1��
С�µļ������Ϊ��CO2������ 10 g + 100g-106.7g=3.3g -------1��
�⣺��̼��Ƶ�����Ϊx���Ȼ��������Ϊy
CaCO3+2HCl=CaCl2+H2O+CO2����
100 73 44
x y 3.3g
x��3.3g = 100��44 x=7.5g��
y��3.3g = 73��44 y=5.5g��--------------------------1��
ͨ������ã�
̼��Ƶ���������Ϊ75% -------------------------1��
�����������������Ϊ5.5% ------------------------1��
��3��С�ռ������õĵ��Dz�������δ���ʵ�ʵ��Dz�������С��4��3g
��С�¼������õ����ݡ�3��3g���а����˷�Ӧ����������ӷ��˵��Ȼ����������������ʵ�����ɵĶ�����̼��������С��3��3g��----------------------------------1��
������57% ------------------------------------------------------------1��
С�µļ������Ϊ��CO2������ 10 g + 100g-106.7g=3.3g -------1��
�⣺��̼��Ƶ�����Ϊx���Ȼ��������Ϊy
CaCO3+2HCl=CaCl2+H2O+CO2����
100 73 44
x y 3.3g
x��3.3g = 100��44 x=7.5g��
y��3.3g = 73��44 y=5.5g��--------------------------1��
ͨ������ã�
̼��Ƶ���������Ϊ75% -------------------------1��
�����������������Ϊ5.5% ------------------------1��
��3��С�ռ������õĵ��Dz�������δ���ʵ�ʵ��Dz�������С��4��3g
��С�¼������õ����ݡ�3��3g���а����˷�Ӧ����������ӷ��˵��Ȼ����������������ʵ�����ɵĶ�����̼��������С��3��3g��----------------------------------1��
��Ϊ��������������ʹ����ʯ��ˮ����ǣ����Ը�����Ϊ������̼���壮���������غ㶨�ɣ��ɷ�Ӧǰ������������ܺ͵IJ�ֵ���������Ӧ�ų�������̼���������ɶ�����̼�����������ݷ�Ӧ�Ļ�ѧ����ʽ���������������������̼��Ƶ�������������øü�������̼��Ƶ�����������

��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
�����Ŀ
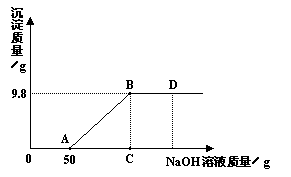
 + C2H2��������X��һ�ּ��X�Ļ�ѧʽΪ ��
+ C2H2��������X��һ�ּ��X�Ļ�ѧʽΪ ��