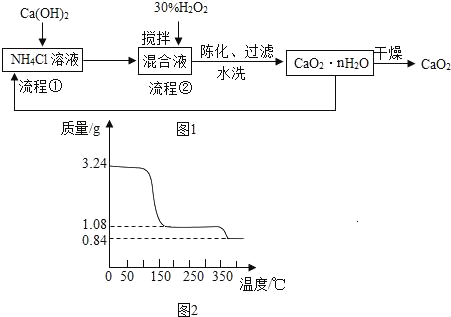
��Ŀ����
����Ŀ����AgNO3��Cu(NO3)2��Zn(NO3)2�����Һ�м���һ���������ۣ���Ӧֹͣ����ˣ��������м���ϡ���ᣬ�����ݲ�����������˵����ȷ����( )
����Һ��һ����Ag��Cu
����Һ��һ����Ag��������Cu��һ��û��Fe��Zn
����Һ��һ����Fe(NO3)2��������AgNO3��Cu(NO3)2��Zn(NO3)2
����Һ��һ����Fe(NO3)2��Zn(NO3)2��������AgNO3��Cu(NO3)2
����Һ��һ����Fe(NO3)2��Zn(NO3)2��һ��û��AgNO3��������Cu(NO3)2
����Һ��������ԭ�����Һ��������ȼ����ˣ�
A. �٢ڢ�B. �٢ۢ�C. �ڢܢ�D. �ܢݢ�
���𰸡�C
��������
���������м���ϡ���ᣬ�����ݲ�����˵������ȫ��Ӧ����һ������������Ӧ���������������������������һ����Ag����һ����Cu����ѡ��˵������ȷ��
���������м���ϡ���ᣬ�����ݲ�����˵������ȫ��Ӧ����һ������������Ӧ�����������������������ܺ�����ͭ��Ӧ��������������ͭ�����������һ����Ag��������Cu��һ��û��Fe��Zn����ѡ��˵����ȷ��
����Һ��һ����������������Ӧ���ɵ�Fe(NO3)2��û�з�Ӧ��Zn(NO3)2����ѡ��˵������ȷ��
����Һ��һ����������������Ӧ���ɵ�Fe(NO3)2��û�з�Ӧ��Zn(NO3)2��������ʣ�����������û�з�Ӧ������ͭ����ѡ��˵����ȷ��
����Һ��һ����Fe(NO3)2��Zn(NO3)2��������ʣ�����������û�з�Ӧ������ͭ����ѡ��˵������ȷ��
������������������ͭ��Ӧ�Ļ�ѧ����ʽ����������ϵΪ��
![]()
![]()
�����Ϸ�����֪������56������������Ӧʱ������216����������������56������������Ӧʱ������64��������ͭ��������ŷ�Ӧ�Ľ��У���Һ��������С����ѡ��˵����ȷ��
��ѡ��C��

 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�����Ŀ��ijͬѧΪ�˲ⶨ������Ʒ������������������60g ϡ�����3�μ��뵽ʢ��4g ����Ʒ���ձ��У���Ʒ��ֻ������ϡ���ᷴӦ�����������������������˵������ȷ���ǣ�������
ʵ����� �������� | ��1�� | ��2�� | ��3�� |
����ϡ���������/g | 20 | 20 | 20 |
��ַ�Ӧ��ʣ���������� | 2.6 | 1.2 | 0.1 |
A. ��1�κ͵�2�β���������������ͬ
B. ��2�κ͵�3��ʣ������о�����
C. ��3�γ�ַ�Ӧ����Һ������ֻ��FeCl2
D. ������Ʒ��������������Ϊ97.5%