��Ŀ����
����Ŀ����ѧ������ϢϢ���.
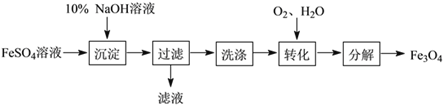
(1)��ͼ1��Ʒ��ʹ�õ���Ҫ�������ںϳɲ��ϵ���________(����ĸ���,��ͬ)�����������ϵ���__________��

(2)�ù�������������������������,�������ʵ��������_______��_____�������ӵĻ�ѧ�仯��д��һ�ַ�ֹ��������ķ���____________��
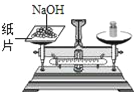
(3)���㶹�ơ���ͼ2��һ����ͳС��,���ݱ����е���Ϣ�ش���������.
Ʒ�� | �㶹�� |
��Ҫԭ�� | �㶹 |
��ҪӪ���ɷ� | �����ʡ����ࡢ�ơ��ơ��ء�þ��Ԫ�� |
�١��㶹�ơ�����Ҫ���е����ʡ������_________����Ӫ���ء�
���㶹�ơ��к��и�Ԫ�أ�����ȱ�Ƴ�������_________(����ĸ���)��
A.ƶѪ֢B.٪��֢C����״���״�D�����Ͳ�
(4)�������������ˮ����Ⱦ����_________(����ĸ���,��ͬ)��
A.��ҵ��ˮֱ���ŷ� B.������ˮ���д������ŷ�
C.��ʹ�ú���ϴ�·� D.����ʹ�û���ũҩ
(5)����̼����һ����������,Ҳ��һ������̬��.���������в����ϡ���̼��Ҫ�����_____
A.��Լֽ��B.����ʹ�û�ʯȼ��
C.�ᳫʹ�ý�Լ�����ͽ��ܲ�ƷD.��������������
(6)�����ѣ�CH3OCH3����һ�ֿ���Ϊ���������Դ�����ȼ��,��������ȫȼ�յĻ�ѧ����ʽΪ______��
(7) ҽԺ��Һ���õ���������ˮ��0.9%���Ȼ�����Һ��ʵ��������������������Ϊ5%���Ȼ�����Һ,�����²���:���ܽ�ڳ��� �ۼ����װƿ���,���ϱ�ǩ����ȷ��˳����________(�����)����ȡ����ˮʱ,�����Ӷ���,����������������_________(����ڡ���С�ڡ�)0.9%�����ø��Ȼ�����Һ��ˮϡ������500����(�ܶ�ԼΪ1g��ml��������ˮ,��Ҫ5%���Ȼ�����Һ________�ˡ�
���𰸡� C AD ���� ˮ Ϳ�͵� ���� D AD B CH3OCH3+3O2![]() 2CO2+3H2O �ۢڢ٢� �� 90
2CO2+3H2O �ۢڢ٢� �� 90
�����������⿼����ǻ�ѧ�������֪ʶ����ɴ��⣬�����������е�֪ʶ������
��1�������˶��������л��ϳɲ����Ƴɵģ���ɺ���ͭ�Ž������������Ƴɵģ���ѡC��AD��
��2��������������ˮ���������棬��ֹ������������������Ϳ�ͣ�
��3���١��㶹�ơ�����Ҫ���е����ʡ���������Σ�
������ȱ�ƻ��������Ͳ�����ѡD��
��4����ҵ��ˮֱ���ŷš�����ʹ�û���ũҩ�����ˮ����Ⱦ��ѡAD��
��5��A����Լֽ�ŷ��ϵ�̼���B������ʹ�û�ʯȼ�ϲ����ϵ�̼���C���ᳫʹ�ý�Լ�����ͽ��ܲ�Ʒ���ϵ�̼���D�������������������ϵ�̼��������ѡB��
��6���������к���̼���⡢������Ԫ�أ���ȫȼ�����ɶ�����̼��ˮ����ѧ����ʽΪ��CH3OCH3 +3O2![]() 2CO2+3H2O��
2CO2+3H2O��
��7������������������Ϊ5%���Ȼ�����Һ�IJ���Ϊ�����㡢�������ܽ⡢װƿ���������ۢڢ٢ܣ����Ӷ���������ȡ��ˮ�������������������������ƫС����ȡ����ˮʱ,�����Ӷ���,����������������С��0.9%������Ҫ5%�Ȼ�����Һ������Ϊx�����У�5%x=500g��0.9% ��x=90g��������Ҫ5%���Ȼ�����Һ90����

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�����Ŀ��ʵ������һƿʧȥ��ǩ�ĺ�ɫ��ĩA��һƿ��ǩ��ȫ����ɫ��ҺB(��ͼ)��

(1)����ʵ��Ա��ʦ�ṩ��Ϣ��A����������5�ַ�ĩ�е�һ�֣�������������ĩ���������̷�ĩ������ͭ��ĩ��̼���Ʒ�ĩ��ľ̿�ۣ���С���ܿ������ɫ�жϷ�ĩ��������__________(�ѧʽ)��
(2)С����֪��A��B��ʲô���ʣ�������������ʵ�飬��������ʵ�鱨�档(�����Լ���������ϡ���ᡢNaOH��Һ��CuSO4��Һ���Թܡ��ƾ��ơ�Сľ��)
ʵ����� | ʵ������ | ʵ����� |
С����ȡ������ĩA����ҺB��һ֧�Թ��л�ϣ��۲����� | ������������ | ��ĩA����ҺB����Ͽ���������������ϣ� �����ۺ�__________(�ѧʽ����ͬ)�� ��___________������ϵ��ж������û�ѧ����ʽ��ʾΪ��___________________________ |
����ȷ����ĩA����ҺB���������Ϣ� |