��Ŀ����
����Ŀ������֪�����ڳ�ʪ�Ŀ����лᷢ����ʴ����ͭƬ�ڳ�ʪ�Ŀ�����Ҳ�����������ͭ������Ϊ̽����ͭ��������ɼ�ͭƬ��ʴ��ԭ����ʵ��С�������һϵ�е�ʵ�顣
��ʵ��һ��
��1��ȡ������ͭ�������Թ��У�����������ϡ���ᣬ������������ͨ�����ʯ��ˮ�У�����ʯ��ˮ����ǣ�ʯ��ˮ����ǵĻ�ѧ����ʽ��_________���ɼ���ͭ�����к���̼Ԫ�ء�
��ʵ�����
Ϊ��һ��̽����ͭ����������ԭ�����������ϵ��ʵ�顣
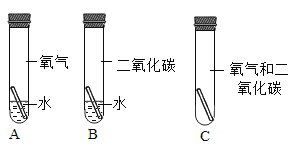
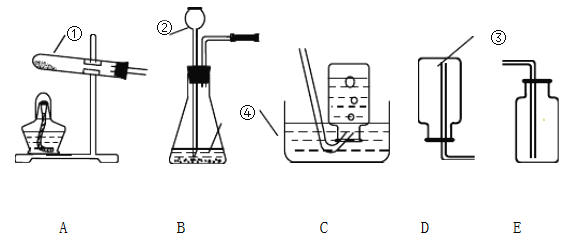
����һ��ʱ�����A��B��C�Թܾ������Ե����ա�
��2�����˸���������ʴԭ�����ó���ͭ����ʴ��ͭ������е�ˮ���������õĽ�����Ľ��ۣ�����ϵ��ʵ����_________����ţ�����֤��������Ǵ���ġ�
��ʵ������
��3���Ľ�A��B��C����ʵ�鶼��ʵ��ͭ����ʴ����Ľ�C�ķ����ǣ�ȡ����Ƥ����ע��������ˮ��Ѹ��������Ƥ������ô���Ľ�A�ķ�����_________��
��ʵ����ۣ�
��4��ͨ������ʵ�鷢�֣�ͭ����ʴ��ͭ��_________���ʹ�ͬ���õĽ����
����չ������
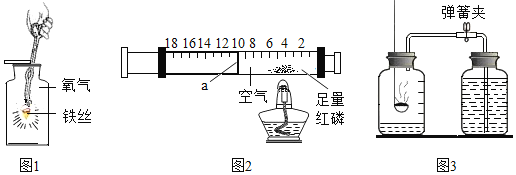
��5��6.4g��ͭת��Ϊ��ͭ������������ԼΪ11.0g������������������ټ���ʱ���Ƶ�ʣ����������Ϊ8.0g����ʣ�������_________�����ţ���
A CuO B Cu2O C Cu(OH)2
���𰸡�CO2 + Ca(OH)2 = CaCO3��+ H2O A ȡ����Ƥ����ͨ������������̼���壬Ѹ��������Ƥ�� ������������̼��ˮ����O2 ��CO2��H2O�� A
��������
��1��ͭ�̺����ᷴӦ�����������̼��������̼�ͺ�ʯ��ˮ��Ӧ����ʽΪ��CO2 + Ca(OH)2 = CaCO3��+ H2O��
��2��ʵ��A��Ӧ����������������һ����������������ʵ��A ��֤��ͭ����ʴ��ͭ������е�ˮ���������õĽ���Ǵ���ģ�
��3���ɸĽ�C��֪����Ľ�ʹ��������ˮ��������̼ͬʱ��ͭ�Ӵ����ʸĽ�A�ķ���Ϊȡ����Ƥ����ͨ������������̼���壬Ѹ��������Ƥ����
��4�������Ͽ�֪ͭ�����Ǹ�������������̼��ˮ��ͬ���õĽ����
��5��ͭ�̼������ɹ��塢������̼��ˮ�����������غ㶨�ɿ�֪ͭ������ͭ��������Ϊ64:80����֪���ɵĹ���Ϊ����ͭ��

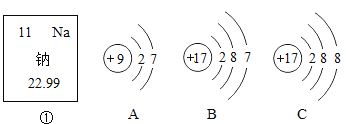
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�