��Ŀ����
��ľ����һ��ũ�ҷʣ�������Ҫ�ɷ���̼��أ���������أ��Ȼ��صȡ���ѧ��ȤС��Ϊ�ⶨij��ľ����Ʒ�е���Ч�ɷ֣�ȡ50g��ľ�����ձ��У����ϵ���������Һ��������30g������Һʱ�����������ݲ�������ʱ�ձ��еIJ������������Ϊ77.8g���������ľ�ҵ������ɷֲ�����Ԫ���Ҳ����ᷴӦ��
�����ش�
��1����ľ�ҵ���Ҫ�ɷ��������������Ϊ____________����ᡢ����Ρ�����
��2����ͼ��ʾ��Ӧ�����зų����������������������Һ�Ĺ�ϵ���ߣ������ͼ����������a����ֵ��a=________________g��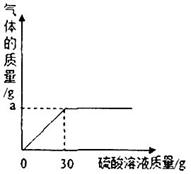
��3�������ľ����Ʒ��̼��ص�������������Ҫ��д��������̣�
��4��ͨ������ʵ�飬��ø�50g��ľ���л��������������Ϊ8.7g���Ȼ��ص�����Ϊ1.49g�����50g��ľ����Ʒ�м�Ԫ�ص�����Ϊ__________g��
��1������2��2.2��3��13.8%��4��8.58
���������������1�����������֪������ľ����һ��ũ�ҷʣ�������Ҫ�ɷ���̼��أ���������أ��Ȼ��صȡ������е�̼��أ���������أ��Ȼ��ؾ����ɽ������Ӻ�������ӹ��ɵĻ�����������ࡣ
��2��a���ʾ������������������ֵ�����������֪��������Ϊ̼��������ᷴӦ���ɵĶ�����̼���塣������Ϊ��50g����ľ�ң�+30g��������Һ����77.8g���������="2.2" g������a="2.2" g��
��3�����������֪����֪��Ϊ���ɶ�����̼��������δ֪��Ϊ��Ʒ��̼��ص�����������
����˼·���ɸ��ݶ�����̼��̼����ڷ�Ӧ�е����������̼��ص���������һ�����������Ʒ�е���������������������£�
�⣺���ľ����Ʒ��̼��ص�����Ϊx
K2CO3+H2SO4=K2SO4+CO2��+H2O
138 44
x 2.2g
138��44=x��2.2g
��ã�x=6.9g
̼��ص���������=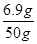 ��100%=13.8%
��100%=13.8%
�𣺲�ľ����Ʒ��̼��ص���������Ϊ13.8%
��4����ľ���еļ�Ԫ����̼��ء�����غ��Ȼ������������еļ�Ԫ�ص������͡��ɷֱ�����������ʵĻ�ѧʽ�������������Ԫ�ص��������������������£�
6.9g̼��أ�K2 CO3���к���Ԫ�ص�����Ϊ��6.9g����
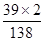 ��100%��=3.9g��
��100%��=3.9g��
8.7g����أ�K2 SO4���к���Ԫ�ص�����Ϊ��8.7g����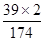 ��100%��=3.9g��
��100%��=3.9g��
1.49g�Ȼ��أ�KCl���к���Ԫ�ص�����Ϊ��1.49g����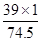 ��100%��=0.78g��
��100%��=0.78g��
��ľ����Ʒ�м�Ԫ�ص�����Ϊ3.9g+3.9g+0.78g=8.58g��
���㣺�ۺϼ���

��5�֣�ijУ����С���ͬѧ�ڲⶨ��MgCl2��NaCl��ɵĹ�����������ʱ������������ʵ�顣ȡ20�˹������200����Һ��ƽ���ֳ��ķݣ�Ȼ��ֱ����һ������������NaOH��Һ������ʵ�����ݼ��±���
| | ʵ��һ | ʵ��� | ʵ���� | ʵ���� |
| ����������Һ������/g | 50 | 50 | 50 | 50 |
| ����NaOH��Һ������/g | 10 | 20 | 30 | 40 |
| ���ɳ���������/g | 1 | m | 2.9 | 2.9 |
��1��m= ��
��2��20gԭ����������Һ������NaOH��Һ��Ӧ������ɳ��������� g��
��3����ʵ�����У���ȫ��Ӧ��������Һ���Ȼ��Ƶ����������Ƕ��٣����������һλС����д��������̣� ��

 CO + H2��
CO + H2��