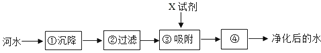
��Ŀ����
����Ŀ��ij��ȤС��ͬѧ��ʵ�����Ʊ�������������������̽��ʵ�顣
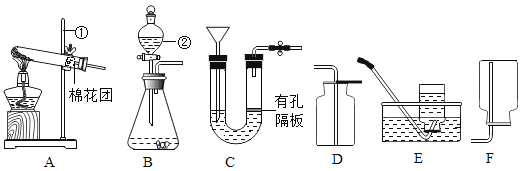
(1)Ϊ̽�����������������طֽ����ʵ�Ӱ�죬��������¶Ա�ʵ�飺
��3.0g KClO3��1.0g MnO2���Ȼ�ϼ���
��x g KClO3��1.0g CuO���Ȼ�ϼ���
����ͬ�¶��£��Ƚ�����ʵ�����O2�Ŀ���������x��ֵӦ��_____��д�����з�Ӧ�Ļ�ѧ����ʽ_____��
(2)̽��Ӱ��˫��ˮ�ֽ����ʵ�ij�����ء�
���ҵ�ʵ�����ݼ�¼���£�
˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2��� | |
�� | 50.0g | 1% | 0.1g | 9 mL |
�� | 50.0g | 2% | 0.1g | 16 mL |
�� | 50.0g | 4% | 0.1g | 31 mL |
��ʵ���У�����O2�����װ����_____(����ĸ)��
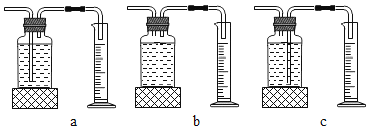
ʵ����ۣ�����ͬ�����£�_____��˫��ˮ�ֽ������Խ�졣
�ڱ�����ͼװ�ý���ʵ�飬ͨ���Ƚ�______Ҳ�ܴﵽʵ��Ŀ�ġ�

���𰸡�3.0 2KClO3![]() 2KCl + 3O2 C ˫��ˮ��Ũ��Խ�� ���ӳ�ʾ���仯�Ŀ���
2KCl + 3O2 C ˫��ˮ��Ũ��Խ�� ���ӳ�ʾ���仯�Ŀ���
��������
��1��Ӧ��ֻ֤�д���������һ�����������3.0��
����ͭΪ����������ʽΪ��2KClO3![]() 2KCl + 3O2�����2KClO3
2KCl + 3O2�����2KClO3![]() 2KCl + 3O2��
2KCl + 3O2��
��2������ˮ���ռ�������ӦΪ�̹ܽ����ܳ�����ѡ��c��
�������п�֪��˫��ˮ��Ũ��Խ��λʱ�������ɵ�����Խ�࣬˫��ˮ�ֽ������Խ�죬���˫��ˮ��Ũ��Խ��
��ͼʾװ����ͨ������װ����������С�ٶȽ����Ƚ�˫��ˮ�ֽ�����ʣ�������ӳ�ʾ���仯�Ŀ�����

����Ŀ�����ж�����֪ʶ�Ĺ��ɣ���ȫ��ȷ��һ���ǣ� ��
A���Ļ������� | B��ѧ���ŵ����� |
�ٸɱ�������Ϊ���壬�������������ӱ�� �ڵ��ˮ�õ�H2��O2����ˮ������Ӻ������ӹ��� ������ɫ�����������������������ڲ�ͣ���˶� | ��CO2����2����ʾһ��������̼���Ӻ���������ԭ�� ��2Na����2����ʾ������ԭ�� �� |
C��ѧ������ | D��ѧ�뻷�� |
�ٿ��õ�ȼ�ķ�����ȥCO2�л��е�������CO �ڸɱ������˹����� ��ϡ�������й㷺����;�����Ƴɶ�����;�ĵ��Դ | �ٳೱ��ˮ������ˮ�帻Ӫ������Ⱦ���� �ڿ�����Ⱦָ��Խ�ߣ���������Խ�� �۽�����ʵ�з�����պ��������� |
A.AB.BC.CD.D