��Ŀ����
����Ŀ����17�֣���������Ҫ�Ľ������ϣ��ڹ�ũҵ������������Ӧ�÷dz��㷺��
I������ұ��
��ҵ������ԭ���Ǹ�������CO����ԭ�����������������л�ԭ��������ش��������⣺
��1����¯�����У���̿�����ó��˿�������һ����̼�⣬���� ��
��2��ij������ÿ������5000t��������80%�ij�����ʯ���ó������Ͽ��ղ�����98%�������������Ƕ��٣�
II��ʵ��̽������ԭ��ij��ѧ��ȤС����ʵ������ģ�ҵ������ԭ������̽��CO��Fe2O3��Ӧ��IJ��ͨ����������֪���������ᣨH2C2O4��������Ũ�����ϼ��Ȼ����һ����̼����Ӧ����ʽΪ��H2C2O4 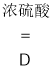 CO��+ CO2��+ H2O��
CO��+ CO2��+ H2O��
��NaOH��Һ�������ն�����̼����Ӧ����ʽΪ��2NaOH+CO2=Na2CO3+H2O
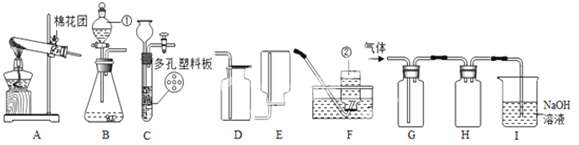
�������£�Ca(OH)2����ˮ���������������ͼ��ʵ��װ�ã����װ�ûش��������⣺
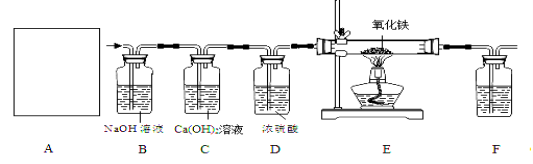
��1��ͼA��������������Ũ������ȡCO�ķ�Ӧװ�ã�����ΪӦѡ����ͼ�е� ����װ�ñ�ţ���
��2��ͼ��װ��C��D�����÷ֱ��� �� ��
��3��Ϊ��֤���������ж�����̼��װ��F�����Լ�Ӧ��������������ʯ��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����װ�������һ������ȱ�ݣ�����Ϊ�� ��
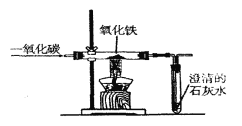
��5��ʵ��ʱȷ��ȡһ������������Fe2O3���尴��ͼ����ʵ�飬��E�й���ȫ����ں���ͨ��COֱ����������ȴ�����õ���ɫ�Ĺ�����뵽������ϡ���ᣬ���ֹ���ȫ���ܽ⣬�������ݲ�����
�������ϣ�a.������������������ϡ�����о���ȫ���ܽ⡣
b.Fe2O3��CO��Ӧ�Ĺ������������������£�
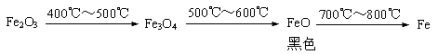
���ݡ������ݡ����룬��ɫ��ĩ�����ǣ��� Fe3O4 ���� �� ��
��6���������� �õ�����ƽ�����������������£�
�������� | ��������������������� | װ��F���������ʵ������� | |
��Ӧǰ | 28.20 g | 33.00 g | 300.0 g |
��Ӧ�� | 32.84 g | 300.4 g |
�����������ݣ���ѡ����Ч�����ݣ��ƶϳ���Ӧ���ɫ����ijɷ֡���д��������̣�
���𰸡�I����1���ṩ��Դ��ά��¯�£�2��2857t
II.��1��������2��֤��![]() �Ƿ��Ѿ�����������ˮ��(����
�Ƿ��Ѿ�����������ˮ��(����
��3��![]() ��
��
��4��ȱ��β������װ��
��5��![]() ��
��![]() ��
��![]()
��6��![]() ��
��
��������I����1������ʱҪ�õ���̿�������������ã�����ʱ��Ҫ���£���̿��������Ӧ���ɶ�����̼��ͬʱ�ų������ȣ��ɹ�������ʹ�ã�����ʱ����һ����̼����ԭ����������̼�������̿��Ӧ��������һ����̼.
��2���躬��98%����������Ϊx��
5000t��80%��![]() = x��98% x=2857t
= x��98% x=2857t
II��ʵ��̽������ԭ��
��1�����ᣨ![]() ����Ũ�����ϼ��Ȼ����һ����̼����Ӧ���ΪҺ�壬����Ҫ���ȣ�����ѡ����װ�ö���
����Ũ�����ϼ��Ȼ����һ����̼����Ӧ���ΪҺ�壬����Ҫ���ȣ�����ѡ����װ�ö���
��2��װ��C֤��![]() �Ƿ��Ѿ�������Ũ���������ˮ�ԣ��������������壻
�Ƿ��Ѿ�������Ũ���������ˮ�ԣ��������������壻
��3�������ʯ��ˮ����������֤������̼���壻����ʽΪ��![]() ��
��
��4��һ����̼�ж�������Ⱦ����������ֱ���ŷ������ȱ��β������װ�ã�
��5������������ϡ���ᣬ���ֹ���ȫ���ܽ⣬�������ݲ�����˵��û�������ɣ���������![]() ��
��![]() ������ˣ���ɫ��ĩ�����ǣ���
������ˣ���ɫ��ĩ�����ǣ���![]() ����
����![]() ����
����![]() ��
��![]() ��
��
��6��������![]() ��������Ϊ��33.00g-28.20g=4.80g��������Ԫ�ص�����Ϊ4.80g��
��������Ϊ��33.00g-28.20g=4.80g��������Ԫ�ص�����Ϊ4.80g��![]() =3.36g����Ԫ�ص�����Ϊ4.80g-3.36g=1.44g����Ӧ����������Ԫ�ص�����Ϊ33.00g-32.84g=0.16g�������跴Ӧ���ɫ����
=3.36g����Ԫ�ص�����Ϊ4.80g-3.36g=1.44g����Ӧ����������Ԫ�ص�����Ϊ33.00g-32.84g=0.16g�������跴Ӧ���ɫ����![]() ������Ԫ�أ���Ԫ��=56x:16y=3.36g����1.44g-0.16g�������x:y=3:4���ʷ�Ӧ���ɫ������
������Ԫ�أ���Ԫ��=56x:16y=3.36g����1.44g-0.16g�������x:y=3:4���ʷ�Ӧ���ɫ������![]()
���������I����������ʱ��Ҫ���£���̿��������Ӧ���ɶ�����̼��ͬʱ�ų���������������������ʯ�к�����������������������������������гɵ�ʽ���㣻II��(1)���ݲ�����Ũ������Ӧ���ڹ�-Һ�����ͷ�����(2)����ʵ��Ŀ�ĺ��Լ������֪��ʯ��ˮ���������̼�Ƿ���ڣ�Ũ�����dz��õĸ������(3)���ݷ�Ӧԭ����д����ʽ��(4)����һ����̼�ж�������Ⱦ�����������Ҫβ������������(5)��Ϊ����������ϡ���ᣬ���ֹ���ȫ���ܽ⣬�������ݲ�����˵��������û���������ݹ���Ϊ��ɫ������(6)���ݲ������з�Ӧǰ������������ó�ʧȥ��Ԫ�ص����������㴿�������к���Ԫ�ص��������ó�ʣ�������������Ԫ�ص������Ƚ��з���.

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�