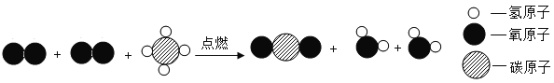
��Ŀ����
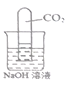
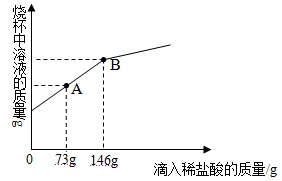
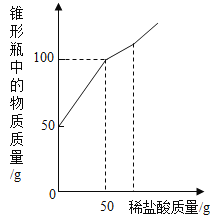
����Ŀ��С����Э����ʦ����ʵ�鴢����ʱ������һƿ��Ŷ�����������ơ�Ϊ������������������������̽����ȡ����������Ʒ11.4g����ƿ�У�����38.6gˮ������ƿ����εμ�14.6%��ϡ���ᣬ����ϡ�������������ƿ�����ʵ�������ϵ��ͼ��ʾ��
��1���������Ʊ��ʵ�ԭ����_____���û�ѧ����ʽ��ʾ����
��2�����������Ʒ�Ӧ��ϡ������Һ������Ϊ_____g��
��3������11.4g����Ʒ���������Ƶ�������_____
��4�����ݼ����������ݻ�������CO2�����������ߣ������б�Ҫ�ı�ע����_____
���𰸡�Ca��OH��2+CO2=CaCO3��+H2O 50 7.4g 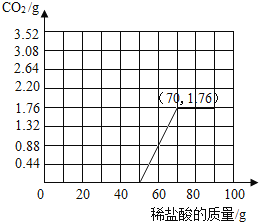
��������
��1������������������еĶ�����̼��Ӧ�������������Ʊ��ʵ�ԭ��Ӧ�Ļ�ѧ����ʽ�ǣ�Ca��OH��2+CO2=CaCO3��+H2O��
��2���������ƺ�̼��ƵĻ���������ᷴӦʱ�����������������ᷴӦ�����������������ᷴӦ���̼����������ᷴӦ����ͼ���֪�����������Ʒ�Ӧ��ϡ������Һ������Ϊ50g��
��3���裺�����ᷴӦ��Ca��OH��2������Ϊx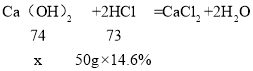
![]() ��ã�x=7.4g��
��ã�x=7.4g��
��4����Ʒ��̼��Ƶ�����=11.4g-7.4g=4g��
�裺4g̼�����ϡ���ᷴӦ����CO2������Ϊy�����������Ϊz��
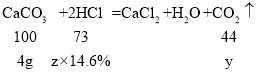
![]()
��ã�y=1.76g z=20g
�����������֪������CO2�����������ߵ�����ǣ�50��0����ת�۵�Ϊ��70��1.76����ͼ������ͼ��
 ��
��

����Ŀ��ѧϰ�������ƵĻ�ѧ���ʺ�С��������ʵ�飺����ʾ���Ȼ�þ��ҺΪ��ɫ������ʹ��̪��Һ��죩
ʵ�� | ʵ����� | ʵ�������Ԥ������ | ���ۻ���� |
�� |
| ����______________ | ������̼���������Ʒ�����ѧ��Ӧ���÷�Ӧ ����ʽΪ__________ |
�� |
| ��Һ����ɫ��Ϊ��ɫ | ���ۣ�_____________________________ |
�� |
| ����ϡ�����δ�۲쵽�����������̪����Һ���ɫ�� | ��������������δ������ѧ��Ӧ |
�� |
| �а�ɫ�������� | ���ɳ�����ԭ���û�ѧ����ʽ���ͣ� _________________ |
�� | 1.��II��III��IV��֧�Թ��е����ʵ���һ���ྻ���ձ��� | �ձ��г��ְ�ɫ���������ã��ϲ���Һ��ɫ | �ϲ���Һ�����ʵijɷ�Ϊ __________ ����ָʾ���⣩ |
2.ȡ�����ϲ���Һ���Թ��У�����_____��Һ����֪̽����Һ�����ʳɷ� | ���ܳ��ֵ�����Ϊ��________________ |
��1���벹�����~�ߵ����ݡ�
��2��С����ΪС��ʵ��I������Ʋ�����˵��������̼���������Ʒ�����ѧ��Ӧ��Ӧ���ĸĽ���_____
��3��С����ΪС��ʵ��III
��4��С��ͬѧ�����ʵ��III������÷�ָ̪ʾ��������������������ƻ�Ϻ����Һ�м���ijЩ���ʣ�������Ӧ���������жϷ�Ӧ�Ƿ��������з�����ȷ����_________
A ����Na2CO3��Һ��������������ݣ���֤����Ӧ������
B ����CuSO4��Һ�������������ɫ��������֤����Ӧ������
C ������ɫʯ����Һ�������Һ����ɫ����֤����Ӧ������