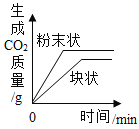
��Ŀ����
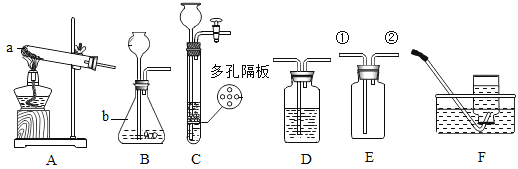
����Ŀ���ס������ֹ������ʵ��ܽ����������ͼ��ʾ���ֽ���֧�ֱ�װ�мס��������ʱ�����Һ���Թܣ��ײ���������δ�ܽ�Ĺ��壩����ʢ��ˮ���ձ��У������ձ��м���һ��Ũ���ᡣ
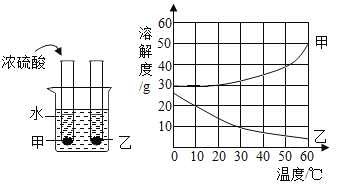
��1��50��Cʱ�������ʵ��ܽ����_____g��
��2���ձ��м���Ũ����ס������Թ��й������ı仯��_____��
��3��30��Cʱ100g�ҵı�����Һ�����ʵ�����Ϊ_____g��������������
��4�������ܽ���������뽫���������ļס��ҵĹ�������һ�η��룬ʹ��Һ�к������������ң����õ��ҵĹ��壬��ľ��������_____��
���𰸡�40 ���٣������� 9 �������ˮ�ܽ⣬�Ƴ�0��ʱ������Һ��Ȼ�����µ�60�棬���ˡ�ϴ�ӡ�����
��������
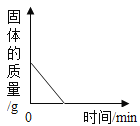
��1����ͼ��֪��50��Cʱ�������ʵ��ܽ����40g������40��
��2��Ũ���������ձ��е�ˮ��ų��������ȣ��ձ��ڵ�Һ���¶����ߣ���ͼ��֪�����ܽ�����¶����߶��������Թ��еļ����ܽ⣬������٣��ҵ��ܽ�����¶����߶���С�����Թ�����Һ�е��ҽᾧ�������Թ��е��ҹ������࣬������٣������ࡣ
��3����ͼ��֪��30��Cʱ�ҵ��ܽ��Ϊ10g����30��Cʱ100g�ҵı�����Һ�����ʵ�����Ϊ100g��![]() ��100%��9%������9��
��100%��9%������9��
��4����ͼ��֪�����ܽ�����¶����߶������ҵ��ܽ�����¶����߶���С�������������ļס��ҵĹ�������һ�η��룬ʹ��Һ�к������������ң����õ��ҵĹ�����Բ������½ᾧ�ķ����õ��ҹ��壬���巽���ǽ������ˮ�ܽ⣬�Ƴ�0��ʱ������Һ��Ȼ�����µ�60�棬���ˡ�ϴ�ӡ�����õ��ҹ��壬��������ˮ�ܽ⣬�Ƴ�0��ʱ������Һ��Ȼ�����µ�60�棬���ˡ�ϴ�ӡ����

����Ŀ���ڻ�ѧ���ϣ���ͬѧ��ȼ�յ���Ѹ�����뵽ʢ��CO2�ļ���ƿ�У��������м���ȼ�գ���Ӧ����ȴ��ƿ���ź�ɫ������ƿ����ճ���Ű�ɫ���ʡ�
��������⣩��ɫ�����Ͱ�ɫ������ʲô?
�����в��룩����Ϊ��ɫ������________����ɫ���ʿ�����Na2O��________��________����ͬѧ����Ϊ��ɫ�������������ơ�
��ͬѧ������Ϊ��ͬѧ�IJ����Ǵ���ģ���������____________________________��
���������ϣ�������Ϊ��ɫ��ĩ������ˮ�����������ƣ�Na2O+H2O===2NaOH
��ʵ��̽������ͬѧ��ɫ���ʽ���ʵ��̽����
ʵ�鷽�� | ʵ����� | ʵ������ | ���� |
����1 | ȡ��Ʒ�������Թ��У���������ˮ������Ʒȫ������ˮ�������м�����ɫ��̪��Һ | ��Һ��ɺ�ɫ | ��ɫ����ΪNa2O |
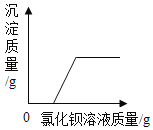
����2 | ��ȡ��Ʒ�������Թ��У���������ˮ������Ʒȫ������ˮ�������м��������CaCI2��Һ | ���ְ�ɫ���� | ��ɫ������_____ |
�ھ���Ƭ�̣�ȡ�ϲ���Һ���Թ��У��μ���ɫ��̪��Һ | ���������� |
����˼���ۣ���ͬѧ��Ϊ����1�õ��Ľ��۲���ȷ����������________________��
���ó����ۣ����ڶ�����̼��ȼ�յĻ�ѧ����ʽΪ___________________________��
����Ŀ����֪�Ȼ��ƺ�����ص��ܽ�������
�¶�/�� | 0 | 20 | 40 | 60 | |
�ܽ��/g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 |
KNO3 | 13.3 | 31.6 | 63.9 | 110 | |
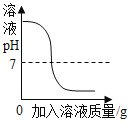
(1)20��ʱ���Ȼ��Ʊ�����Һ����������������_________________%��
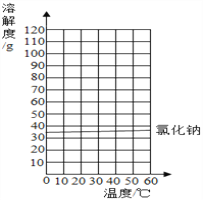
(2)������ͼ�л���KNO3���ܽ�����ߣ�ͼ�����ܽ�����߽����������________________
(3)KNO3�ǻ�ѧ�����е�___���ϣ����ȵ�KNO3Ũ��Һ����������ķ�����________________________ ��