��Ŀ����
����Ŀ����ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ���ش�:
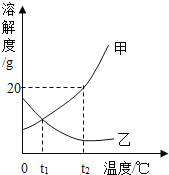
(1)��___________ʱ���ס����������ʵ��ܽ����ͬ��
(2)��t1��C�ı�����Һ��Ϊ��������Һ�ɲ��÷�����__________��
(3) t2��Cʱ�� ��25g����װ��100gˮ���ձ��У���ȫ�ܽ��������Һ����Ϊ_______________��
(4)�����й�˵����ȷ����____________ (�����)��
�ټ��к����������ҿɲ��ý��½ᾧ�ķ����ᴿ��
��t2��Cʱ������Һ��������������������Һ��������������
��t2��Cʱ�����������ס��ҷֱ��ˮ��ɱ�����Һ�����ü���Һ����������ҺС
�ܽ�t2��Cʱ�ס��ұ�����Һ������t1��Cʱ�����üס�����Һ�����������������
�ݽ�t2��Cʱ120g�ı�����Һ������t1��Cʱ��������20g����
���𰸡�t1�� ���ܼ������� 120�� �٢�
��������
�ɼס������ֹ������ʵ��ܽ������ͼ��֪���������ʵ��ܽ�����¶ȵ����߶������ҹ������ʵ��ܽ�����¶ȵ����߶���С��
(1)�ܽ�����ߵĽ����ʾ��ʾ�¶����������ʵ��ܽ����ȣ�����t1��ʱ���ס����������ʵ��ܽ����ͬ��
(2) �������ʵ��ܽ�����¶ȵ����߶�����t1��C�ı�����Һ��Ϊ��������Һ�ɲ��÷����Ǽ��ܼ������¡�
(3) t2��Cʱ���������ʵ��ܽ��Ϊ20g����25g����װ��100gˮ���ձ��У���ȫ�ܽ�ļ����ʵ�����Ϊ20g����������Һ����Ϊ![]() ��
��
(4)���������ʵ��ܽ�����¶ȵ����߶������ҹ������ʵ��ܽ�����¶ȵ����߶���С�����к����������ҿɲ��ý��½ᾧ�ķ����ᴿ�ף�����ȷ��
��t2��Cʱ��δָ����Һ�Ƿͣ������жϼ���Һ��������������������Һ���������������Ĵ�С��ϵ���ʲ���ȷ��
��t2��Cʱ�����ܽ�ȱ��ҵ��ܽ�ȴ��������ס��ҷֱ��ˮ��ɱ�����Һ��������ˮ��������������ˮ������С�����ü���Һ����������ҺС������ȷ��
�ܽ�t2��Cʱ���ס��ұ�����Һ������t1��Cʱ�����ܽ�ȼ�С���ױ�����Һ���о����������õ�����t1��C�ı�����Һ���ҵ��ܽ�����õ�����t1��C�ҵIJ�������Һ��t1��Cʱ���ҵ��ܽ����ȣ����ü���Һ����������������������Һ�����������������ʲ���ȷ��
�ݽ�t2��Cʱ��120g�ı�����Һ�к���������Ϊ![]() ����ˮ������Ϊ
����ˮ������Ϊ![]() ��������t1��Cʱ�����ܽ�ȼ�С���о�������������t1��Cʱ�ܽ�ȴ���0��100gˮ�����ܽ���һ�������ļף����������������С��20g���ʲ���ȷ��
��������t1��Cʱ�����ܽ�ȼ�С���о�������������t1��Cʱ�ܽ�ȴ���0��100gˮ�����ܽ���һ�������ļף����������������С��20g���ʲ���ȷ��
��ѡ�٢ۡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��������;�㷺����ṹ�����ʵ��ǻ�ѧ����Ҫ�о����ݡ�
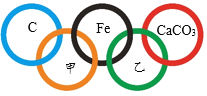
��1����ͼ������ԭ�ӽṹʾ��ͼ������˵������ȷ����______��
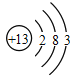
A��ԭ�ӵ�������Ϊ13
B�ڻ���������ͨ����+3��
C���ǵؿ��к�������Ԫ��
D�������������������������õĵ�����
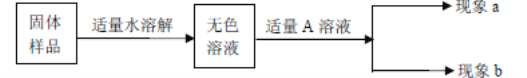
��2��ij��ѧС����һ����AgNO3��Cu(NO3)2�����Һ��������ͼʵ�飬 ������ҺA����B�ijɷֽ����˷�����ʵ��̽����
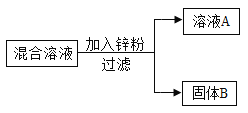
��������⣩��ҺA�е����ʿ�������Щ��
���������룩��ֻ��Zn(NO3)2����Zn (NO3)2��AgNO3����Zn (NO3)2��Cu(NO3)2����Zn (NO3)2��AgNO3��Cu(NO3)2
���������ۣ��������IJ�����__________�����ţ�����������_________________��
��ʵ��̽����������ٳ�����ͨ������ʵ���ȷ������B�ijɷ֣��뽫�±���д������
ʵ�鲽�� | ���� | �йط�Ӧ�Ļ�ѧ����ʽ |
ȡ��������B���μ�________________ | �����ݲ��� | ____________________ |
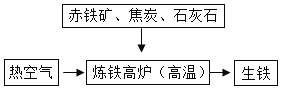
��3����ͼ�ǹ�ҵ����ʾ��ͼ�����У���̿��������ȼ���ṩ������_______________���������ɵĻ�ѧ����ʽΪ________________��
��4����73 g��������Ϊ20%��������127g����������Һǡ����ȫ�кͣ��Լ��㷴Ӧ��������Һ�����ʵ���������__________��
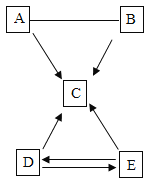
����Ŀ��Ϊ̽������Ļ�ѧ���ʣ�ij��ѧС����������ʵ�飺
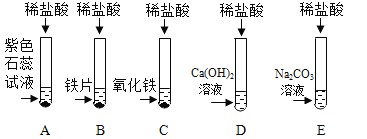
��1��C �Թ�����������Ӧ�Ļ�ѧ����ʽΪ ____________ ��
��2������Ӧ�� D��E �Թ��еķ�Һ����һ���ྻ���ձ��У��۲쵽�ձ����������ݲ��������а�ɫ�������֡����ձ� �еĻ������ˣ��õ���ɫ��������ɫ��Һ��ͬѧ�Ƕ���Һ�����ʵijɷֽ���̽���� (�������)��Һ�����ʵijɷ���ʲô��
(��������)����һ��NaCl��
�������NaCl �� CaCl2��
��������__________________��
�����ģ�NaCl��Ca(OH)2�� HCl ��
��ʦָ���������Ǵ���ģ�ԭ����_______________�� (���ʵ��)�����ʵ�鱨�档
ʵ�鲽�� | ʵ������ | ʵ����� |
��ȡ������Һ���Թ��У��μ�������̼������Һ | _____________ | ����������� |
��ȡ������Һ���Թ��У��μ�__________(������) | �������� | ���������� |