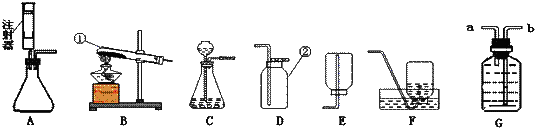
��Ŀ����
С��Ҫ��100g��������Ϊ8%������������Һ��������Ҷ����ǩ����ͼ1������������������Һ��ʵ�����ʾ��ͼ��

��1����ȡ����Ҫ��ˮӦѡ�� ���10 mL������50 mL����100 mL������Ͳ��
��2��ָ��ͼ1�е�һ��������� ���ô����������ĺ���� ��
��3��������ͼ1��ʾ����ű�ʾ������Һ�IJ���˳�� ��
��4�����ƺõ�����������ҺҪ�ܷⱣ��ԭ���ǣ� ���û�ѧ����ʽ��ʾ����
��5������ȷ���Ƶ�����������Һ��μ���30gϡ�����У���Ӧ�����е�pH �仯��ͼ2��ʾ�ٵ���������������Һ����Ϊc����ʱ��Һ������Ϊ ��������Ϊbg10%������������Һ�����ʱ������Һ�� �ԣ���ᡱ��������С�������bΪ20g��ȷ���Ƶ���Һ��������ʱ������Һ�����ʵ�����������

��1����ȡ����Ҫ��ˮӦѡ��
��2��ָ��ͼ1�е�һ���������
��3��������ͼ1��ʾ����ű�ʾ������Һ�IJ���˳��
��4�����ƺõ�����������ҺҪ�ܷⱣ��ԭ���ǣ�
��5������ȷ���Ƶ�����������Һ��μ���30gϡ�����У���Ӧ�����е�pH �仯��ͼ2��ʾ�ٵ���������������Һ����Ϊc����ʱ��Һ������Ϊ
���㣺һ������������������Һ������,������-������ƽ,�й��������������ļ���,�кͷ�Ӧ����Ӧ��,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��Һ����Һ���ܽ��,�������� ���ͨ��
��������1������100g��������Ϊ10%������������Һ����������������������һ������Һ�Ŀ��⣬Ҫȷ����ѡ����Ͳ�����ȱ���֪����Ҫȡˮ�������
��2��ʹ����ƽʱ��Ҫ��ѭ���������ԭ��ʹ����Ͳ����ʱ��Ҫʹ������Һ�尼Һ�����ʹ�����ˮƽ�����ӻ��Ӷ�����ɶ�����ȷ����ϸ�۲�ͼ������ȷ������Ĵ𰸣�
��3��������Һ��һ�㲽��Ϊ���㡢�������ܽ⡢װƿ��ǩ��ŵȣ����Ա���Ĵ𰸺���������ѡ��
��4��ҩƷ���ʱҪע���ֹ���ʣ����������������������̼������Ӧ������Ӧ�ܷⱣ�森
��5����ͼ��֪����������������Һ����Ϊc����ʱ��Һ�Լ��ԣ�����bg8%%������������Һʱ�������ǡ����ȫ��Ӧ����ʱ��Һ�����ԣ�����Ϊbg10%������������Һ���������ƻ���ʣ�࣬����Һ�Լ��ԣ�
��2��ʹ����ƽʱ��Ҫ��ѭ���������ԭ��ʹ����Ͳ����ʱ��Ҫʹ������Һ�尼Һ�����ʹ�����ˮƽ�����ӻ��Ӷ�����ɶ�����ȷ����ϸ�۲�ͼ������ȷ������Ĵ𰸣�
��3��������Һ��һ�㲽��Ϊ���㡢�������ܽ⡢װƿ��ǩ��ŵȣ����Ա���Ĵ𰸺���������ѡ��
��4��ҩƷ���ʱҪע���ֹ���ʣ����������������������̼������Ӧ������Ӧ�ܷⱣ�森
��5����ͼ��֪����������������Һ����Ϊc����ʱ��Һ�Լ��ԣ�����bg8%%������������Һʱ�������ǡ����ȫ��Ӧ����ʱ��Һ�����ԣ�����Ϊbg10%������������Һ���������ƻ���ʣ�࣬����Һ�Լ��ԣ�
����⣺��1������100g��������Ϊ10%������������Һ�������������Ƶ�����Ϊ100g��10%=10g����Ҫˮ������Ϊ100g-10g=90g����ˮһ�����������ķ���������ˮ�����Ϊ90mL��ѡ����Ͳʱ��Ӧ�ó�����ȡҺ���������ҽӽ���ԭ�����Ա����Ϊ��100mL
��2��ʹ����ƽʱ��Ҫ��ѭ���������ԭ���������������ڸ�ʴ��ҩƷ������ڲ��������г�����ʹ����Ͳ����ʱ��Ҫʹ������Һ�尼Һ�����ʹ�����ˮƽ�����ӻ��Ӷ�����ɶ�����ȷ��
��3���ù�������������Һ��һ�㲽��Ϊ���㡢�������ܽ⡢װƿ��ǩ��ţ����Ա����Ϊ���ܢ٢ۢݢڣ�
��4�������������������̼������ѧ��Ӧ�����ʣ����Ա����Ϊ��2NaOH+CO2=Na2CO3+H2O
��5����ͼ��֪����������������Һ����Ϊc����ʱ��Һ�Լ��ԣ�����Ϊ�������������ƺ��������������ᷴӦ���ɵ��Ȼ��ƣ�����bg8%%������������Һʱ�������ǡ����ȫ��Ӧ����ʱ��Һ�����ԣ�����Ϊbg10%������������Һ���������ƻ���ʣ�࣬����Һ�Լ��ԣ�
�⣺���ʱ������Һ�����ʵ�����Ϊx
HCl+NaOH�TNaCl+H20
40 58.5
20g��8% x
=
x=23.4g ��ʱ��Һ�����ʵ���������Ϊ
��100%��46.1%
�𣺴�ʱ������Һ�����ʵ���������46.1%
�ʴ�Ϊ����1��100mL
��2�����������Ʒ���ֽ�ϳ���������ˮʱ���Ӷ���������ȡ��ˮƫ�ࣻ
��3���ܢ٢ۢݢ�
��4��2NaOH+CO2=Na2CO3+H2O
��5��46.1%
��2��ʹ����ƽʱ��Ҫ��ѭ���������ԭ���������������ڸ�ʴ��ҩƷ������ڲ��������г�����ʹ����Ͳ����ʱ��Ҫʹ������Һ�尼Һ�����ʹ�����ˮƽ�����ӻ��Ӷ�����ɶ�����ȷ��
��3���ù�������������Һ��һ�㲽��Ϊ���㡢�������ܽ⡢װƿ��ǩ��ţ����Ա����Ϊ���ܢ٢ۢݢڣ�
��4�������������������̼������ѧ��Ӧ�����ʣ����Ա����Ϊ��2NaOH+CO2=Na2CO3+H2O
��5����ͼ��֪����������������Һ����Ϊc����ʱ��Һ�Լ��ԣ�����Ϊ�������������ƺ��������������ᷴӦ���ɵ��Ȼ��ƣ�����bg8%%������������Һʱ�������ǡ����ȫ��Ӧ����ʱ��Һ�����ԣ�����Ϊbg10%������������Һ���������ƻ���ʣ�࣬����Һ�Լ��ԣ�
�⣺���ʱ������Һ�����ʵ�����Ϊx
HCl+NaOH�TNaCl+H20
40 58.5
20g��8% x
| 40 |
| 20g��8% |
| 58.5 |
| x |
x=23.4g ��ʱ��Һ�����ʵ���������Ϊ
| 23.4g |
| 20g+30g |
�𣺴�ʱ������Һ�����ʵ���������46.1%
�ʴ�Ϊ����1��100mL
��2�����������Ʒ���ֽ�ϳ���������ˮʱ���Ӷ���������ȡ��ˮƫ�ࣻ
��3���ܢ٢ۢݢ�
��4��2NaOH+CO2=Na2CO3+H2O
��5��46.1%
���������⿼��������������������һ������Һ��Ҫ����ѡ�����õ�ʵ��������ʵ�鲽�裬����ע��ʵ���������ȷʵ����������⣮

��ϰ��ϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A�������½������ǹ��� |
| B������������ʴ����ǿ |
| C����ͭ�����������������Ͻ� |
| D��ͭ��Ӳ�ȱȻ�ͭ�� |