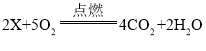��Ŀ����
����Ŀ����1�����ʷ����ǻ�ѧ�о��ij��÷���������Ҫ��Ӷ�����̼�������������ơ����ᡢ�������ơ��Ȼ�����ѡ����ʵ����ʣ����仯ѧʽ����ո��С�
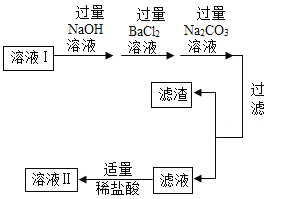
���ܹ������������嵥��_______________________________��
��θ�����Ҫ�ɷ�_______________________________��
��һ�ֳ�����ʳƷ�����_______________________________��
��һ�ִ���������ˮ�ļ�_______________________________��
��һ��������ˮ����_______________________________��
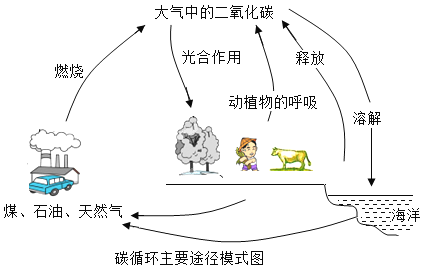
��2������˿��֮·������һ��һ·���ij��飬�ٽ��˶����������Ļ�����Ľ�������һ�� һ·������˿��֮·���ô�������21���ͺ���˿��֮·���ļ�ơ���˿��֮·�����й���˿��Ҷ����Ʒ�����������ֽ������ı�ʯ�����ϡ���Դ����Դ�����й���
�����ʲ�Ҷ�и���ά����C,�仯ѧʽΪC6H8O6��ά����C��_______________________________��Ԫ�����,����̼����Ԫ�ص�������Ϊ_______________________________����ά����C��,______________________________Ԫ�ص��������������Ԫ�����ƣ���
�ڱ�ʯ���������صĿ��Σ��ɷָ��ӣ�������ʯ����Ҫ�ɷ���Al2O3,������Ԫ�صĻ��ϼ�Ϊ_______________________________��
����Դ��������һ��һ·������Ҫ���ݣ����������ܵ���ʯ�ͺ���Ȼ�������й�����Ȼ������Ҫ�ɷ��Ǽ��飨CH4������д��������ȫȼ�յĻ�ѧ����ʽ_______________________________��
���𰸡�O2 HCl CaO Ca��OH��2 AgCl 3 3:4 �� +3 ![]()
��������
��1������һ��Ԫ����ɵĴ������ǵ��ʣ��ܹ������������嵥��������
��θ�����Ҫ�ɷ����
�۳��г����������Ũ���ᡢ�������ơ������ƣ����������Ƴ�����ʳƷ�������
���ڳ����ļ��У��������������ã�����������������ˮ��
�ݳ������������У��Ȼ��������ᱵ��̼��Ƶȣ�
��2�������ʲ�Ҷ�и���ά����C���仯ѧʽΪC6H8O6��ά����C��̼���⡢������Ԫ�����,����̼����Ԫ�ص�������Ϊ��6��12��:��6��16��=3:4����ά����C�У�̼���⡢��Ԫ�ص�������Ϊ��6��12��:��8��1��:��6��16��=9:1:12������Ԫ�ص������������
�ڻ�������Ԫ�صĻ��ϼ۴�����Ϊ�㣬������ʯ����Ҫ�ɷ���Al2O3����Ԫ����-2�ۣ�������Ԫ�صĻ��ϼ�Ϊ+3��
����Ȼ������Ҫ�ɷ��Ǽ��飨CH4����������ȫȼ�ռ�����������ڵ�ȼ���������ɶ�����̼��ˮ����ѧ����ʽCH4 + 2O2 ![]() CO2 + 2H2O��
CO2 + 2H2O��

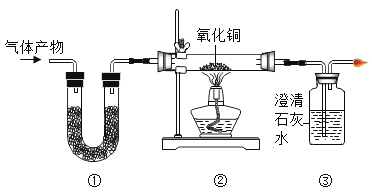
����Ŀ��ʵ������ȡ����ʱ��Ҫ��һЩװ������ͼ��ʾ������Ũ��������ˮ�������������ش��������⡣
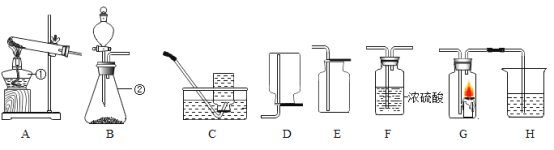
��1��д��������ŵ��������ƣ���_____
��2��ʵ������ȡ�����Ͷ�����̼�ķ���װ�ö���ѡ��Bװ�õ�ԭ����_____��װ����ʹ�÷�Һ©�����ŵ���_____��
��3����Ҫ�õ��������������ѡ��װ�õ�����˳��Ϊ��B��_____��_____������ĸ��ţ���
��4��ij��ȤС���ͬѧ����B��G��Hװ�ý���ʵ�顣��ʵ��ʱGװ��������ȼ�ո����ң�Hװ������Һ����ǣ���Bװ���з�Ӧ�Ļ�ѧ����ʽΪ_____��
��5��ʵ�����Ʊ����ռ�������̼ʱ�����±�ѡȡҩƷ����ʵ�飬ȡ�������Ĵ���ʯ�����������У����ʲ����ᷴӦ��������������̼�����ʱ��仯��������ͼ��ʾ��
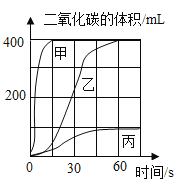
ʵ���� | ҩƷ |
I | ��״����ʯ��10%������Һ |
�� | ��״����ʯ��7%������Һ |
�� | ����ʯ��ĩ��7%������Һ |
ͼ�б���Ӧʵ��_____��ѡ���������������������ü����߶�Ӧ��ҩƷ��������_____��