��Ŀ����
����Ŀ��ijʵ���о�С����������ᾧ��ֽ�IJ��ﲢ�ⶨ�䴿�������ʲ�������Ӧ����
���������ϣ�
�ٲ��ᾧ�壨H2C2O42H2O��100�濪ʼʧˮ��101.5���ۻ���189�����ҷֽ⡣
����ˮ����ͭ��ˮ�ɰ�ɫ�����ɫ��
�ۼ�ʯ�Ҽȿ�����ˮҲ�������� CO2
�ס�����ͬѧ���ò�ͬ�ķ���������ᾧ��ֽ�IJ��ﲢ�ⶨ�䴿�ȣ�
��1������ɲ�����
����������裩
����һ���ֽ������ CO2��H2O ��������ֽ������ CO�� H2O
���������ֽ������_____��
��ʵ����ƣ�
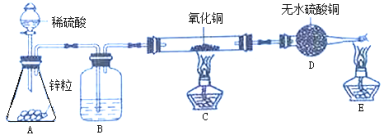
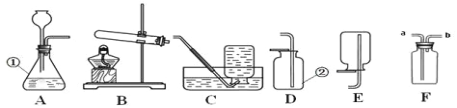
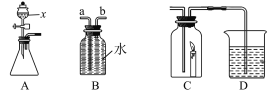
��. ��ͬѧ������ʵ��������ͼ��ijЩ����װ������ȥ��ʵ������ҩƷ���ޣ�����̽����
��2������a ��������_____��
��3���ü��Ȳ��ᾧ��ķ�����ȡ���壬Ӧ��ѡ������巢��װ����_____�����ţ���
��4����ͬѧʵ������װ�á�G����ʯ�ң���F��C��CuO ���壩��F������ CO���� F ��ʢװ���Լ���_____��֤������ CO ��������_____��
��. ��ͬѧ��װ�ý����˸Ľ�����Ƶ���װ������ͼ��ʾ����������Ϊ�ײ��������Թܣ�
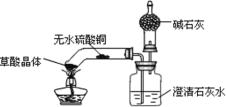
��ʵ����̣�
��ͬѧ�����Թܺ�۲쵽��ˮ����ͭ�ɰ�ɫ�����ɫ�������ʯ��ˮ����ǣ������ɵ�����ͨ����ʯ�Һ��ڸ���ܵļ��촦��ȼ����������ȼ�ա�ͨ����һ�����飬ȷ��ȼ�յ�����Ϊ CO��
��ʵ����ۣ�
��5������_____����ȷ�ģ�д�����ᾧ�����ȷֽ�Ļ�ѧ����ʽ��_____��
�����ȼ��㣩
��ͬȡ��Ʒa g��ͨ��ʵ���ò�����CO ����b g������ᴿ�ȵı���ʽΪ_____��
����˼�����ۣ�����ʵ�������������Ա�����װ�ã�ָ����ͬѧ��Ƶ�װ���ŵ���_____��
���𰸡�CO2��CO�� H2O�ձ�B����ʯ��ʯ����ʯ��ˮ�������H2C2O4![]() H2O+CO2��+CO��
H2O+CO2��+CO��![]() ��β�������˴�����ֹһ����̼��Ⱦ����
��β�������˴�����ֹһ����̼��Ⱦ����
��������
��1��������Ӧ�ð�������һ�Ͳ�����IJ������Ӧ�CO2��CO�� H2O��
��2������aΪʵ���ҳ��������ձ���
��3�����ȹ���ʱ�Թܿ�Ӧ����������б����ѡ��Bװ�ã�
��4��һ����̼��ֱ�Ӽ��飬���ǿ��Խ�һ����̼ת��Ϊ������̼���м��飬����F��װ���dz���ʯ��ʯ����ʵ������Ϊ����ʯ��ˮ����ǡ�
��5��ʵ������к���ˮ��������̼��һ����̼���������ȷ������ֽ����ˮ��������̼��һ����̼����ʽ��H2C2O4![]() H2O+CO2��+CO��;
H2O+CO2��+CO��;
���ݻ�ѧ����ʽ�躬�в���X���ɷ���ʽ��

���֮����ͬѧ��ʵ��װ�ö�β���Ĵ����Ϻã���������ɫ��ѧ�����