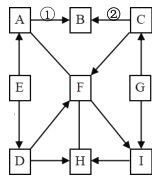
��Ŀ����
����Ŀ��(2018����������ģ��)�ڻ�ѧ����ʵ���У�ijͬѧ��������2 mL 10%���Ȼ�����Һ�еμ�����������Һ�������˰�ɫ������
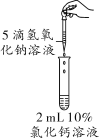
С��ͬѧ�dz�����Ȥ����̽������
��������⣩���ɵİ�ɫ������ʲô��
����������裩
������������Һ���ʣ����ɵİ�ɫ������________��
������������ҺŨ�Ƚϴ����ɵİ�ɫ�������������ơ�
������ʵ�飩��ͬѧ��Բ���ٽ���ʵ�顣
����������Һ���ʵĻ�ѧ��Ӧ����ʽ��__________________________��
ʵ�鲽�� | ʵ������ | ʵ����� |
������Һ���ˣ�ȡ�����������ձ��У���������ˮ | ________ | ����ٲ����� |
��ͬѧ���������������һ�ַ���֤������ٲ�������ȡ�����������Թ��У��μ�����ϡ���ᣬ���۲쵽________________��������֤�������롣
��ͬѧ��Բ���ڽ���ʵ��
��ʵ�������ȡ5֧�Թܣ������зֱ����2 mL 10%�Ȼ�����Һ���ٷֱ�μ�5��Ũ��Ϊ0.4%��0.5%��1%��2%��4%������������Һ��ʵ���������±���
����������ҺŨ��/% | �Ƿ���� |
0.4 | ���������� |
0.5 | ����� |
1 | ���Ի��� |
2 | ���Ի��� |
4 | ���Ի��� |
����������ۣ�(1)��ͬѧʵ��֤������ڳ������ܷ���ֻ���������������ҺŨ�ȵĹ�ϵ��______________________________��
(2)����������ɵij������������Ƶ�ԭ����________________________��
��ʵ����չ����������������Һ�Ƿ���ʣ�������ʵ���֪�������ҩƷ����ʹ���Ȼ�����Һ������ѡ��һ�ּ�����Լ�________��
���𰸡�̼���(��CaCO3) 2NaOH��CO2=Na2CO3��H2O ������ȫ�ܽ� �����ܽ⣬�����ݲ��� ����������ҺŨ��Խ��Խ���ײ������� ������������ˮ�������ɵ������������϶�ʱ����ˮ�в���ȫ���ܽ�ͱ���˳���(��������) ϡ����(��ϡ����Ⱥ�������)
��������
��