题目内容
生活中处处有化学。
(1)下表为某品牌燕麦片的主要成分。
表中没有列出的营养素是 ,人体中若缺少表中的 元素(写元素符号)会造成骨质疏松。
(2)小明用洗涤剂清洗餐具上的油污,是利用了洗涤剂的 作用。
(3)小李在室温下开启一瓶未经冰冻的碳酸性饮料时,发现有大量的气泡从罐内冒出。这种现象说明气体的溶解度与 有关。
(4)小马欲粗略测定家里所用白醋中醋酸的质量分数(假设白醋中的酸都看作醋酸,醋酸的化学式为CH3COOH),在玻璃杯中加入20.0 g碳酸钙粉末,再倒入200.0 g白醋。反应停止后,碳酸钙有剩余,称得玻璃杯中的固体和液体总质量为217.8 g。醋酸与碳酸钙反应的化学方程式为:2CH3COOH + CaCO3 =(CH3COO)2Ca + CO2↑+ H2O,若不考虑醋酸与水的挥发及二氧化碳的溶解,所测白醋中CH3COOH的质量分数为 。(写出计算过程)
(1)下表为某品牌燕麦片的主要成分。
| 每100g含有的营养成分 | 糖类 | 油脂 | 蛋白质 | 维生素C | 钙 | 钠 | 锌 |
| 7.6g | 7.8g | 7.4g | 18mg | 201mg | 30.8mg | 8.1mg |
(2)小明用洗涤剂清洗餐具上的油污,是利用了洗涤剂的 作用。
(3)小李在室温下开启一瓶未经冰冻的碳酸性饮料时,发现有大量的气泡从罐内冒出。这种现象说明气体的溶解度与 有关。
(4)小马欲粗略测定家里所用白醋中醋酸的质量分数(假设白醋中的酸都看作醋酸,醋酸的化学式为CH3COOH),在玻璃杯中加入20.0 g碳酸钙粉末,再倒入200.0 g白醋。反应停止后,碳酸钙有剩余,称得玻璃杯中的固体和液体总质量为217.8 g。醋酸与碳酸钙反应的化学方程式为:2CH3COOH + CaCO3 =(CH3COO)2Ca + CO2↑+ H2O,若不考虑醋酸与水的挥发及二氧化碳的溶解,所测白醋中CH3COOH的质量分数为 。(写出计算过程)
(1)水(H20) Ca (2)乳化 (3)压强
(4) 二氧化碳的质量= 200g+20g-217.8g = 2.2g;所测白醋中CH3COOH的质量分数为3% 。
(4) 二氧化碳的质量= 200g+20g-217.8g = 2.2g;所测白醋中CH3COOH的质量分数为3% 。
试题分析:(1)人体的六大营养物质为水、蛋白质、脂肪、维生素、糖类、无机盐离子,人体缺钙元素会造成骨质疏松;(2)洗涤剂清洗餐具上的油污是利用了其乳化作用;(3)明气体的溶解度与压强有关;(4)二氧化碳的质量= 200g+20g-217.8g = 2.2g
解:设CH3COOH的质量为x。
2CH3COOH + CaCO3 = (CH3COO)2Ca + CO2↑+H2O
120 44
x 2.2g
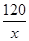 =
= 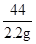 , x=6.0 g
, x=6.0 g 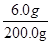 ×100% = 3%
×100% = 3% 答:所测白醋中CH3COOH的质量分数为3% 。
点评:这是化学反应联系方程式的计算题,这种题目是每年中考的压轴题,必考题,这类题目的难点在于把坐标曲线表示的意义和题干联系起来。

练习册系列答案
 综合自测系列答案
综合自测系列答案
相关题目
 Na2CO3+H2O+CO2↑.现取NaHCO316.8g,在敞口容器中加热到质量不再改变为止,减少的质量为( )
Na2CO3+H2O+CO2↑.现取NaHCO316.8g,在敞口容器中加热到质量不再改变为止,减少的质量为( )