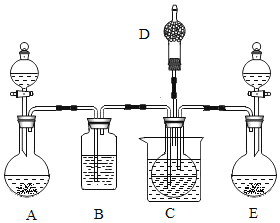
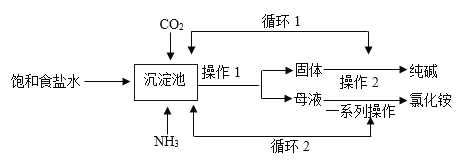
��Ŀ����
����Ŀ��ˮ����Һ�����������������������ش�����壬����ˮ��ԴԽ��Խ�������ǵ����ӣ�ij��ѧ��ȤС���ѧУ������ˮ��״������������о����飺
��1��ȡ��ˮ�������ú���ˣ�ʵ���ҹ���ʱ��Ҫ�IJ����������ձ���©����______��
��2������______�����ˮ����Ӳˮ������ˮ���ճ������н�Ӳˮת��Ϊ��ˮ�ķ�����______��
��3������ˮ���������м��������أ�K2FeO4������ɱ��ʱ��������_________�����������仯��������ѧ�仯�����������������Ԫ�صĻ��ϼ�Ϊ______��
��4���������������ˮ����Ⱦ����_____��������ţ�
�ٹ�ҵ��ˮ��괦�����ŷ� �����ⶪ���Ͼɵ��
���ú���ϴ�·�ϴ�º�ֱ���ŷ���ˮ �ܺ���ʹ�û��ʺ�ũҩ
��5��ʵ���ҵ��ˮ�Ļ�ѧ����ʽ_________________��
��6�����Ȼ��ƹ����ˮ����6%���Ȼ�����Һ80g������ȡˮʱ���Ӷ�����������������ȷ�������Ƶ��Ȼ�����Һ��������������____6% (����>����<������ = ��)
���𰸡������� ����ˮ ��У��������У� ��ѧ�仯 +6 �ڢ� 2H2O![]() 2H2����O2�� >
2H2����O2�� >
��������
��1������ʱ��Ҫ�IJ����������ձ���©������������
��2�����÷���ˮ�����ˮ����Ӳˮ������ˮ�����в�����ĭ�϶������ˮ����ĭ�϶����Ӳˮ���ճ������н�Ӳˮת��Ϊ��ˮ�ķ�������У�
��3��������أ�K2FeO4������ɱ��ʱ�����������ɣ����ڻ�ѧ�仯���ɻ������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ0��K2FeO4��֪�������������Ԫ�صĻ��ϼ�Ϊ+6��
��4���ٹ�ҵ��ˮ��괦�����ŷŲ������ˮ����Ⱦ���ʴ���
�����ⶪ���Ͼɵ�ػ����ˮ����Ⱦ������ȷ��
���ú���ϴ�·�ϴ�º�ֱ���ŷ���ˮ���ˮ����Ⱦ������ȷ��
�ܺ���ʹ�û��ʺ�ũҩ�������ˮ����Ⱦ���ʴ���
��5�����ˮ������������������Ӧ�Ļ�ѧ����ʽΪ2H2O![]() 2H2����O2����
2H2����O2����
��6����ȡˮʱ���Ӷ����������ˮ��������٣��������Ƶ��Ȼ�����Һ����������������6%��

����Ŀ���ܽ���ǽ����Һ����������Ҫ���ݡ�
I.�����ܽ�����߽����������: (M��N�������ᾧˮ)
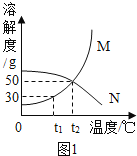
��1��t1��ʱ����20g M����50gˮ�У�����ܽ⣬�γ�_________(����͡������͡�)��Һ����Һ������Ϊ_____________g.�����¶Ȳ��䣬�����Һ���ټ���10gˮ��ֽ��裬��Һ����������������_______(��������С�����䡱) ;
��2��M�����к�������N���ʣ�����__________�����ᴿM����(����½ᾧ���������ᾧ��) ;
��3��t2��ʱ����25gN����50gˮ�У���ȫ�ܽ⡣������߸���Һ���������������������������____________��
II.���ݱ�����ʵ������:
�¶�/��C | 20 | 30 | 50 | 80 | 90 | |
�ܽ��/g | KNO3 | 31.6 | 45.8 | 85.5 | 100 | 169 |
K2CO3 | 110 | 114 | 121 | 126 | 139 | |
ijKNO3��Ʒ�к�������K2CO3,���ᴿ������ͼ2(����������ˮ������û�б仯):
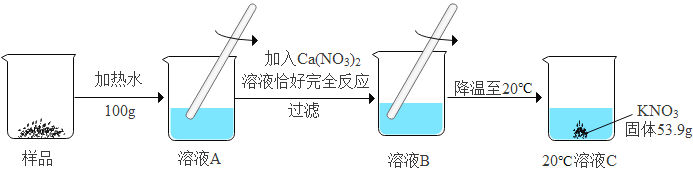
��1��ͼ2����ҺC��__________(����͡������͡�)��Һ; .
��2����Ʒ�м�Ԫ�ص�����Ϊ__________g (�����������).