��Ŀ����
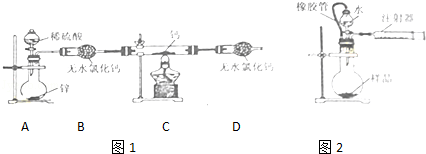
����һ����������������ɳ�Ȳ��������ʺ�����Na2SO4��MgCl2��CaCl2�ȿ��������ʵĴ�����Ʒ��ijʵ��С�����û�ѧʵ���ҳ��������Դ�����Ʒ�����ᴿ���ᴿ�������£�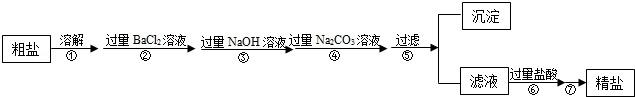
������ᴿ����ش��������⣮
��1������ߵIJ�������Ϊ ��
��2����д��ʵ�鲽��������漰�Ļ�ѧ����ʽ ��
��3��������м�����������Ŀ���� ��
��4������ںͲ���� ������ԡ������ԡ����ߵ��������� ��
��5�����鲽�����Na2CO3��Һ�ѹ����ķ����� ��
��6���ӵ�ʳ�������Ϣ��ͼ��ʾ��
ʳ���еĵ���أ�KIO3�������������£����Խ��⻯�أ�KI����ɵ⣨I2������ѧ����ʽ���£�
KIO3+5KI+6HCl=6KCI+3I2+3H2O
����װ�е⻯�غ͵��ۻ��Һ���Թ��У�����ϡ���Ὣ��Һ�ữ���ټ���ʳ�Σ���ʳ�����е⻯�أ������ʳ�κ��ʵ������ ��
��Сǿͬѧ���ⶨ�ӵ����е�Ԫ�ص�����������ʵ�鲽�����£�ȡ10gʳ����Ʒ���Թ��м�ˮ�ܽ⣬�������KI�ĺ͵��ۻ����Һ���ٵ���ϡ���Ὣ��Һ�ữʹ���ַ�Ӧ������Һ�����ԣ������Թ��еμ������������Һ��Na2S2O3����������ѧ��Ӧ����ʽΪ��2Na2S2O3+I2�TNa2S4O6+2NaI
��������������Ϊ0.237%Na2S2O3��Һ2gʱ��I2ǡ�÷�Ӧ��ȫ��ͨ�������жϸ�ʳ����Ʒ�Ƿ�ϸ���֪Na2S2O3����Է�������Ϊ158����д��������̣���
��1�������ᾧ
��2��BaCl2+Na2SO4�TBaSO4��+2NaCl��
��3����ȥ�������������ƺ�̼���ƣ�
��4�������ԣ�����ߵ�������ȥ�������Ȼ�����
��5��ȡ�ϲ���Һ���Թ��У��μ��Ȼ�����Һ��������ְ�ɫ������˵��̼������Һ�Ѿ�����
��6�����ϸ�
���������������1������ߵIJ�������Ϊ�����ᾧ��
��������ᾧ��
��2��ʵ�鲽������漰�Ȼ����������Ʒ�Ӧ���Ȼ����������Ʒ�Ӧ���������ᱵ���Ȼ��ƣ���ѧ����ʽΪ��BaCl2+Na2SO4�TBaSO4��+2NaCl��
���BaCl2+Na2SO4�TBaSO4��+2NaCl��
��3��������м�����������Ŀ���dz�ȥ�������������ƺ�̼���ƣ�
�����ȥ�������������ƺ�̼���ƣ�
��4������ߵ�������ȥ�������Ȼ�����
��������ԣ�����ߵ�������ȥ�������Ȼ�����
��5�����鲽�����Na2CO3��Һ�ѹ����ķ����ǣ�ȡ�ϲ���Һ���Թ��У��μ��Ȼ�����Һ��������ְ�ɫ������˵��̼������Һ�Ѿ�������
���ȡ�ϲ���Һ���Թ��У��μ��Ȼ�����Һ��������ְ�ɫ������˵��̼������Һ�Ѿ�������
��6����KIO3+5KI+6HCl=6KCI+3I2+3H2O��2Na2S2O3+I2�TNa2S4O6+2NaI��֪��I��Na2S2O3�Ķ�Ӧ��ϵΪ��I��6Na2S2O3��
��10gʳ����Ʒ�е�Ԫ�ص�����ΪX��
2g0.237%��Na2S2O3��Һ��Na2S2O3������Ϊ��2g��0.237%=0.00474g��
I��6Na2S2O3��
127 948
X 0.00474g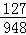 =
=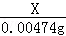
X=0.000635g��
10gʳ����Ʒ�е�Ԫ�ص�������0.000635g��1000gʳ����Ʒ�е�Ԫ�ص�����Ϊ��0.000635g��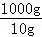 =0.0635g=63.5mg��
=0.0635g=63.5mg��
��ͼ�е���Ϣ�Ƚϣ���ʳ����Ʒ���ϸ�
���㣺�Ȼ���������ᴿ�����˵�ԭ������������Ӧ�ã��εĻ�ѧ���ʣ����ݻ�ѧ��Ӧ����ʽ�ļ��㣻������ۡ������ǵķ����뵰���ʵ����ʣ�
������������Ҫ��������ᴿ�Ĺ��̺��ݻ�ѧ����ʽ���м��㣬����ʱһ��Ҫ������ϸ�����������

 ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д� 3Fe+4CO2����100t��Fe3O480%�Ĵ�����ʯ��������ұ��������4%���������٣����𰸱���һλС����
3Fe+4CO2����100t��Fe3O480%�Ĵ�����ʯ��������ұ��������4%���������٣����𰸱���һλС����
 CaH2��
CaH2��