��Ŀ����
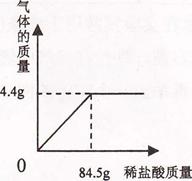
��7�֣�Ϊ����ij������ͭ��ͭ��ɵĻ����������ͭ��������������ȡ3�ݸû�����20g���ֱ������뵽50g��100g��150gijϡ�����У��������ʵ���������±���
����ʵ�鼰�й����ݽ��з�������㣺
��1��20g��Ʒ�к�����ͭ������Ϊ______g����Ʒ������ͭ����������Ϊ________��
��2���������õ�ϡ�������ʵ����������Ƕ��٣���д��������̣�
��3�����ڢ���������ˣ�����Һ�еμ�10%��NaOH��Һ�������ڸû����Һ�м���10%NaOH��Һ�����������ɳ��������仯��ϵ������ͼ�����������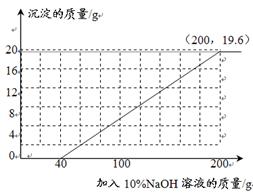
| | ��I�� | �ڢ��� | �ڢ��� |
| ϡ���������/g | 50 | 100 | 150 |
| �ܽ���������/g | 10.0 | 16.0 | 16.0 |
��1��20g��Ʒ�к�����ͭ������Ϊ______g����Ʒ������ͭ����������Ϊ________��
��2���������õ�ϡ�������ʵ����������Ƕ��٣���д��������̣�
��3�����ڢ���������ˣ�����Һ�еμ�10%��NaOH��Һ�������ڸû����Һ�м���10%NaOH��Һ�����������ɳ��������仯��ϵ������ͼ�����������

��1��16��80%����ÿ��1�֣�
��2���裺�����������������Ϊx��0.5�֣�
CuO + H2SO4="==" CuSO4+H2O ��0.5��
80 98
10g 50g��x
x =24.5%��1�֣�
������ϡ�����������������Ϊ24.5%����0.5�֣�
��3����2�֣��������40��0����1�֣����۵㣨200��19.6����1�֣�
��2���裺�����������������Ϊx��0.5�֣�
CuO + H2SO4="==" CuSO4+H2O ��0.5��
80 98
10g 50g��x
x =24.5%��1�֣�
������ϡ�����������������Ϊ24.5%����0.5�֣�
��3����2�֣��������40��0����1�֣����۵㣨200��19.6����1�֣�
��1����Ʒ�е�����ͭ�����ᷴӦ��ͭ�������ᷴӦ����һ��ʵ��50gϡ�����ܽ�������������10.0g���ڶ���ʵ����100gϡ�����ܽ�������������16.0g���ɴ˵ó����ۣ���һ��ʵ����㣬�ڶ���ʵ����ʣ������ͭ��ȫ��Ӧ�����Ӷ������20g��Ʒ�к�����ͭ���������������������Ĺ�ʽ�����Ʒ������ͭ������������
��2�����Ϸ�����һ��ʵ������ȫ��Ӧ����˿����õ�һ��ʵ���е����ݺ�CuO+H2SO4�TCuSO4+H2O ������õ�ϡ�������ʵ�����������
��3�����Ϸ����ڶ���ʵ��������ʣ�࣬������Һ�еμ�����������Һʱ�����ǿɿ�������������Һ�Ⱥ��ᷴӦ�ٺ�����ͭ��Ӧ�����õڶ���ʵ���е����ݺ�CuO+H2SO4�TCuSO4+H2O �����ʣ���ϡ������������Ӷ��õ�������꣬Ȼ��������CuSO4+2NaOH=Cu��OH��2��+Na2SO4���������ͭ��Ӧ������������Һ�������Լ����ɵij����������Ӷ��õ��۵����꣮
��2�����Ϸ�����һ��ʵ������ȫ��Ӧ����˿����õ�һ��ʵ���е����ݺ�CuO+H2SO4�TCuSO4+H2O ������õ�ϡ�������ʵ�����������
��3�����Ϸ����ڶ���ʵ��������ʣ�࣬������Һ�еμ�����������Һʱ�����ǿɿ�������������Һ�Ⱥ��ᷴӦ�ٺ�����ͭ��Ӧ�����õڶ���ʵ���е����ݺ�CuO+H2SO4�TCuSO4+H2O �����ʣ���ϡ������������Ӷ��õ�������꣬Ȼ��������CuSO4+2NaOH=Cu��OH��2��+Na2SO4���������ͭ��Ӧ������������Һ�������Լ����ɵij����������Ӷ��õ��۵����꣮

��ϰ��ϵ�д�
�����Ŀ