��Ŀ����
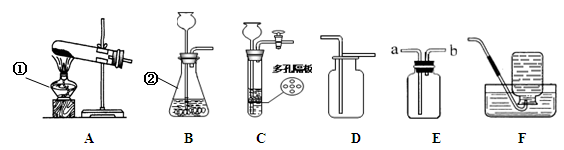
��10�֣���������ͼ��ʾ��ʵ��װ��ͼ����Ҫ��ش��й����⣺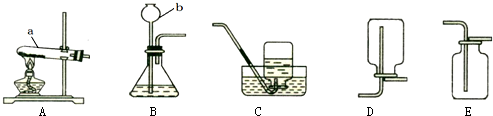
��1��д��ͼ�д��б�����������ƣ�a ��b ��
��2����Bװ����ȡ����ʱ���������Ļ�ѧ��Ӧ����ʽΪ ��
��3��ʵ�����ø��������ȡ���ռ������������Ӧѡ��װ�� ������ĸ�������������ռ����ķ����ǣ� ��
��4�������dz��л�ѧ������Ҫ��ʵ���ʵ��װ�á�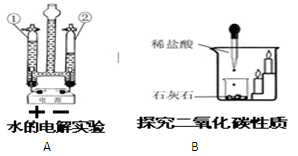
�� Aʵ����߲����ܢ� �в����������� ��?
��Bʵ���������Ϩ��˵��������̼���е������� ��
д��B�з�Ӧ�Ļ�ѧ����ʽ ��
��1��a �Թ� ��b ����©������2�� 2H2O2MnO22H2O+O2�� ����3�� AE�� �Ѵ����ǵ�ľ������ƿ�ڣ�ľ����ȼ�����������۲��Ƿ�ȼ������4��Z�� ���� ��?�ڲ�֧��ȼ�գ�����ܶȺͲ���ȼ���۷֣��� CaCO3+2HCl==CaCl2+H2O+CO2��.
����

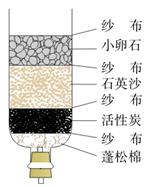
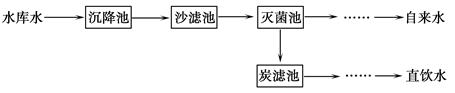
��6�֣�ˮ������ͨ�����������֮һ��
��1����ˮ���кܶ��֡����С�ˮ�����ڴ�������� ������ĸ��ţ���
| A����ˮ | B������ˮ | C����Ȫˮ | D������ˮ |
��3��ˮ����Ҫ���ܼ��ͻ���ԭ�ϡ��ȼҵ�Ա���ʳ��ˮΪԭ�ϻ���ռ��
������Ʒ����Ӧԭ��Ϊ��2NaCl + 2H2Oͨ��2NaOH + H2�� + Cl2�� ��
��20��ʱ��NaCl ���ܽ����36 g�����¶��£�����ʳ��ˮ���������ܼ�������Ϊ ��
���ռ�����ڴ�������й©����Ӧ�Ļ�ѧ����ʽΪ ��
��4��ˮ�ڻ�ѧʵ���о�����Ҫ���á�����˿���ڳ�ʪ�Ŀ����У���ͼ��ʾ����һ��ʱ��۲쵽������Һ���½������ܿ�������ð�����ر� K������͵�����Һ���������½���ԭ�� ��


 ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ��� ����ʾ��ԭ�ӡ�
����ʾ��ԭ�ӡ�